Microvascular endothelial cells derived from spinal cord promote spinal cord injury repair
- PMID: 37621772
- PMCID: PMC10444976
- DOI: 10.1016/j.bioactmat.2023.06.019
Microvascular endothelial cells derived from spinal cord promote spinal cord injury repair
Abstract
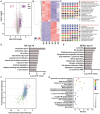
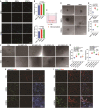
Neural regeneration after spinal cord injury (SCI) closely relates to the microvascular endothelial cell (MEC)-mediated neurovascular unit formation. However, the effects of central nerve system-derived MECs on neovascularization and neurogenesis, and potential signaling involved therein, are unclear. Here, we established a primary spinal cord-derived MECs (SCMECs) isolation with high cell yield and purity to describe the differences with brain-derived MECs (BMECs) and their therapeutic effects on SCI. Transcriptomics and proteomics revealed differentially expressed genes and proteins in SCMECs were involved in angiogenesis, immunity, metabolism, and cell adhesion molecular signaling was the only signaling pathway enriched of top 10 in differentially expressed genes and proteins KEGG analysis. SCMECs and BMECs could be induced angiogenesis by different stiffness stimulation of PEG hydrogels with elastic modulus 50-1650 Pa for SCMECs and 50-300 Pa for BMECs, respectively. Moreover, SCMECs and BMECs promoted spinal cord or brain-derived NSC (SNSC/BNSC) proliferation, migration, and differentiation at different levels. At certain dose, SCMECs in combination with the NeuroRegen scaffold, showed higher effectiveness in the promotion of vascular reconstruction. The potential underlying mechanism of this phenomenon may through VEGF/AKT/eNOS- signaling pathway, and consequently accelerated neuronal regeneration and functional recovery of SCI rats compared to BMECs. Our findings suggested a promising role of SCMECs in restoring vascularization and neural regeneration.
Keywords: Microvascular endothelial cells; Neural regeneration; NeuroRegen scaffold; Spinal cord injury.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Shen H., Fan C., You Z., Xiao Z., Zhao Y., Dai J. Advances in biomaterial-based spinal cord injury repair. Adv. Funct. Mater. 2022;32(13) doi: 10.1002/adfm.202110628. - DOI
LinkOut - more resources
Full Text Sources

