Recent progress in high-performance environmental impacts of the removal of radionuclides from wastewater based on metal-organic frameworks: a review
- PMID: 37622006
- PMCID: PMC10445089
- DOI: 10.1039/d3ra04177h
Recent progress in high-performance environmental impacts of the removal of radionuclides from wastewater based on metal-organic frameworks: a review
Abstract
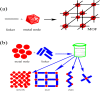
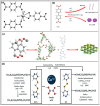
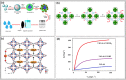
The nuclear industry is rapidly developing and the effective management of nuclear waste and monitoring the nuclear fuel cycle are crucial. The presence of various radionuclides such as uranium (U), europium (Eu), technetium (Tc), iodine (I), thorium (Th), cesium (Cs), and strontium (Sr) in the environment is a major concern, and the development of materials with high adsorption capacity and selectivity is essential for their effective removal. Metal-organic frameworks (MOFs) have recently emerged as promising materials for removing radioactive elements from water resources due to their unique properties such as tunable pore size, high surface area, and chemical structure. This review provides an extensive analysis of the potential of MOFs as adsorbents for purifying various radionuclides rather than using different techniques such as precipitation, filtration, ion exchange, electrolysis, solvent extraction, and flotation. This review discusses various MOF fabrication methods, focusing on minimizing environmental impacts when using organic solvents and solvent-free methods, and covers the mechanism of MOF adsorption towards radionuclides, including macroscopic and microscopic views. It also examines the effectiveness of MOFs in removing radionuclides from wastewater, their behavior on exposure to high radiation, and their renewability and reusability. We conclude by emphasizing the need for further research to optimize the performance of MOFs and expand their use in real-world applications. Overall, this review provides valuable insights into the potential of MOFs as efficient and durable materials for removing radioactive elements from water resources, addressing a critical issue in the nuclear industry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Efficient and irreversible capture of strontium ions from aqueous solution using metal-organic frameworks with ion trapping groups.Dalton Trans. 2019 Mar 14;48(10):3284-3290. doi: 10.1039/c9dt00434c. Epub 2019 Feb 18. Dalton Trans. 2019. PMID: 30776035
-
Metal-organic frameworks as superior porous adsorbents for radionuclide sequestration: Current status and perspectives.J Chromatogr A. 2021 Oct 11;1655:462491. doi: 10.1016/j.chroma.2021.462491. Epub 2021 Aug 25. J Chromatogr A. 2021. PMID: 34482010 Review.
-
A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents.Chemosphere. 2023 Jan;311(Pt 1):136933. doi: 10.1016/j.chemosphere.2022.136933. Epub 2022 Oct 21. Chemosphere. 2023. PMID: 36280122 Review.
-
High-performance removal of radionuclides by porous organic frameworks from the aquatic environment: A review.J Environ Radioact. 2021 Nov;238-239:106710. doi: 10.1016/j.jenvrad.2021.106710. Epub 2021 Sep 1. J Environ Radioact. 2021. PMID: 34481100 Review.
-
Nanoengineered metal-organic framework for adsorptive and photocatalytic mitigation of pharmaceuticals and pesticide from wastewater.Environ Pollut. 2022 Sep 1;308:119690. doi: 10.1016/j.envpol.2022.119690. Epub 2022 Jun 27. Environ Pollut. 2022. PMID: 35772620 Review.
Cited by
-
Progressive Insights into Metal-Organic Frameworks and Metal-Organic Framework-Membrane Composite Systems for Wastewater Management.Molecules. 2024 Apr 3;29(7):1615. doi: 10.3390/molecules29071615. Molecules. 2024. PMID: 38611894 Free PMC article. Review.
-
Simultaneous removal of caesium and strontium using different removal mechanisms of probiotic bacteria.Sci Rep. 2024 Apr 1;14(1):7630. doi: 10.1038/s41598-024-57678-8. Sci Rep. 2024. PMID: 38561437 Free PMC article.
-
Recent Advances in Metal-Organic Frameworks and Their Derivatives for Adsorption of Radioactive Iodine.Molecules. 2024 Sep 3;29(17):4170. doi: 10.3390/molecules29174170. Molecules. 2024. PMID: 39275018 Free PMC article. Review.
References
-
- Belmonte Z. J. A. Prasetyo Y. T. Benito O. P. Liao J.-H. Susanto K. C. Young M. N. Persada S. F. Nadlifatin R. Nucl. Eng. Technol. 2023;23:1738–5733.
-
- Berndes G. Hoogwijk M. Van den Broek R. Biomass Bioenergy. 2003;25:1–28.
-
- Bilgen S. Renewable Sustainable Energy Rev. 2014;38:890–902.
-
- Breyer C. Bogdanov D. Aghahosseini A. Gulagi A. Child M. Oyewo A. S. Farfan J. Sadovskaia K. Vainikka P. Prog. Photovolt.: Res. Appl. 2018;26:505–523.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

