A pilot study on spatial hearing in children with congenital unilateral aural atresia
- PMID: 37622080
- PMCID: PMC10446965
- DOI: 10.3389/fped.2023.1194966
A pilot study on spatial hearing in children with congenital unilateral aural atresia
Abstract
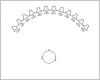
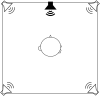
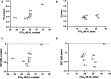
Despite normal hearing in one ear, individuals with congenital unilateral aural atresia may perceive difficulties in everyday listening conditions typically containing multiple sound sources. While previous work shows that intervention with bone conduction devices may aid spatial hearing for some children, testing conditions are often arranged to maximize any benefit and are not very similar to daily life. The benefit from amplification on spatial tasks has been found to vary between individuals, for reasons not entirely clear. This study has sought to expand on the limited knowledge on how children with unilateral aural atresia recognize speech masked by competing speech, and how horizontal sound localization accuracy is affected by the degree of unilateral hearing loss and by amplification using unilateral bone conduction devices when fitted before 3 years of age. In a within-subject, repeated measures design, including 11 children (mean age = 7.9 years), bone conduction hearing device (BCD) amplification did not negatively affect horizontal sound localization accuracy. The effect on speech recognition scores showed greater inter-individual variability. No benefit from amplification on a group level was found. There was no association between age at fitting and the benefit of the BCD. For children with poor unaided sound localization accuracy, there was a greater BCD benefit. Unaided localization accuracy increased as a function of decreasing hearing thresholds in the atretic ear. While it is possible that low sound levels in the atretic ear provided access to interaural localization cues for the children with the lowest hearing thresholds, the association has to be further investigated in a larger sample of children.
Keywords: BCD; UCHL; bone conduction device; early fitting; sound localization; speech recognition; unilateral aural atresia; unilateral conductive hearing loss.
© 2023 Josefsson Dahlgren, Engmér Berglin, Hultcrantz and Asp.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Contribution of spectral pinna cues for sound localization in children with congenital unilateral conductive hearing loss after hearing rehabilitation.Hear Res. 2020 Jan;385:107847. doi: 10.1016/j.heares.2019.107847. Epub 2019 Nov 17. Hear Res. 2020. PMID: 31786443
-
Differing Bilateral Benefits for Spatial Release From Masking and Sound Localization Accuracy Using Bone Conduction Devices.Ear Hear. 2022 Nov-Dec 01;43(6):1708-1720. doi: 10.1097/AUD.0000000000001234. Epub 2022 May 19. Ear Hear. 2022. PMID: 35588503 Free PMC article.
-
Improved directional hearing of children with congenital unilateral conductive hearing loss implanted with an active bone-conduction implant or an active middle ear implant.Hear Res. 2018 Dec;370:238-247. doi: 10.1016/j.heares.2018.08.006. Epub 2018 Aug 26. Hear Res. 2018. PMID: 30174182 Free PMC article.
-
Unexplained Variation in Benefit of Treatment of Congenital Unilateral Aural Atresia: A Review of the Literature.Audiol Neurootol. 2021;26(5):295-302. doi: 10.1159/000512245. Epub 2021 Feb 10. Audiol Neurootol. 2021. PMID: 33567425
-
Sound Localization and Lateralization by Bilateral Bone Conduction Devices, Middle Ear Implants, and Cartilage Conduction Hearing Aids.Audiol Res. 2021 Sep 30;11(4):508-523. doi: 10.3390/audiolres11040046. Audiol Res. 2021. PMID: 34698075 Free PMC article. Review.
References
-
- Priwin C, Jönsson R, Magnusson L, Hultcrantz M, Granström G. Audiological evaluation and self-assessed hearing problems in subjects with single-sided congenital external ear malformations and associated conductive hearing loss. Int J Audiol. (2007) 46(4):162–71. 10.1080/14992020601077984 - DOI - PubMed
LinkOut - more resources
Full Text Sources

