Remote monitoring of cardiac implantable electronic devices and disease management
- PMID: 37622591
- PMCID: PMC10451003
- DOI: 10.1093/europace/euad233
Remote monitoring of cardiac implantable electronic devices and disease management
Abstract
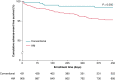
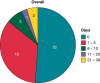
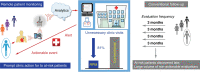
This reviews the transition of remote monitoring of patients with cardiac electronic implantable devices from curiosity to standard of care. This has been delivered by technology evolution from patient-activated remote interrogations at appointed intervals to continuous monitoring that automatically flags clinically actionable information to the clinic for review. This model has facilitated follow-up and received professional society recommendations. Additionally, continuous monitoring has provided a new level of granularity of diagnostic data enabling extension of patient management from device to disease management. This ushers in an era of digital medicine with wider applications in cardiovascular medicine.
Keywords: Defibrillators; Follow-up; Guidelines; Patient monitoring; Remote monitoring.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: N.V.: Research/Consulting for Abbott, Biotronik, and Impulse Dynamics; Pacemate; Implicity; and JB Research/Consulting Boston Scientific, Zoll; F.B.: Medtronic, Biotronik, Biosense Webster, Impulse Dynamics, Novartis, Orion, Boehringer, Astra Zeneca, and Pfizer; H.B.: Abbott, Biotronik, Boston Scientific, Medtronic, and Microport. G.H.: (paid to Heart Center Leipzig): Biosense and Boston Scientific; D.L.: none; Y.M.: none; R.P.R.: minor consultancy fees for Abbott and Dompé Farmaceutici S.p.A.; J.C.N.: institutional research grants from the Novo Nordisk Foundation outside this work.
Figures


References
-
- Niraj V, Pedro B. Automatic home monitoring of patients with cardiac implantable electronic devices—the setting of a new standard. Europace 2013;15:i1–i2. http://europace.oxfordjournals.org/content/15/suppl_1.toc. - PubMed
-
- Santini M. Remote monitoring and the twin epidemics of AF and CHF. Europace 2013;15:i47–8. - PubMed
-
- Varma N, Brugada P. Automatic remote monitoring: milestones reached, paths to pave. Europace 2013;15:i69–71. - PubMed
-
- Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AMet al. . HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations: developed in partnership with the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA); and in collaboration with the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), the Heart Failure Association of ESC (HFA), and the Heart Failure Society of America (HFSA). Endorsed by the Heart Rhythm Society, the European Heart Rhythm Association (a registered branch of the ESC), the American College of Cardiology, the American Heart Association. Europace 2008;10:707–25. - PubMed
-
- Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada Jet al. . ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace 2012;14:278–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

