Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19
- PMID: 37622872
- PMCID: PMC10452855
- DOI: 10.3390/bios13080786
Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19
Abstract
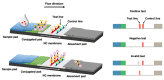
The COVID-19 pandemic has spurred demand for efficient and rapid diagnostic tools that can be deployed at point of care to quickly identify infected individuals. Existing detection methods are time consuming and they lack sensitivity. Point-of-care testing (POCT) has emerged as a promising alternative due to its user-friendliness, rapidity, and high specificity and sensitivity. Such tests can be conveniently conducted at the patient's bedside. Immunodiagnostic methods that offer the rapid identification of positive cases are urgently required. Quantum dots (QDs), known for their multimodal properties, have shown potential in terms of combating or inhibiting the COVID-19 virus. When coupled with specific antibodies, QDs enable the highly sensitive detection of viral antigens in patient samples. Conventional lateral flow immunoassays (LFAs) have been widely used for diagnostic testing due to their simplicity, low cost, and portability. However, they often lack the sensitivity required to accurately detect low viral loads. Quantum dot (QD)-based lateral flow immunoassays have emerged as a promising alternative, offering significant advancements in sensitivity and specificity. Moreover, the lateral flow immunoassay (LFIA) method, which fulfils POCT standards, has gained popularity in diagnosing COVID-19. This review focuses on recent advancements in QD-based LFIA for rapid POCT COVID-19 diagnosis. Strategies to enhance sensitivity using QDs are explored, and the underlying principles of LFIA are elucidated. The benefits of using the QD-based LFIA as a POCT method are highlighted, and its published performance in COVID-19 diagnostics is examined. Overall, the integration of quantum dots with LFIA holds immense promise in terms of revolutionizing COVID-19 detection, treatment, and prevention, offering a convenient and effective approach to combat the pandemic.
Keywords: COVID-19; detection; lateral flow immunoassay; performance; point-of-care testing; quantum dots.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO . Statement on the Second Meeting of the International Health Regulations Emergency Committee regarding the Outbreak of Novel Coronavirus (2019-nCoV) WHO; Geneva, Switzerland: 2005.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

