Colorimetric and Label-Free Optical Detection of Pb2+ Ions via Colloidal Gold Nanoparticles
- PMID: 37622904
- PMCID: PMC10452563
- DOI: 10.3390/bios13080819
Colorimetric and Label-Free Optical Detection of Pb2+ Ions via Colloidal Gold Nanoparticles
Abstract
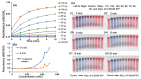
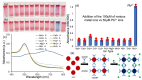
The detection of the lead heavy metal (Pb) in water is crucial in many chemical processes, as it is associated with serious health hazards. Here, we report the selective and precise colorimetric detection of Pb2+ ions in water, exploiting the aggregation and self-assembly mechanisms of glutathione (GSH)-functionalized gold nanoparticles (GNPs). The carboxyl functional groups are able to create coordination complexes with Pb2+, inducing aggregation amongst the GSH-GNPs in the presence of Pb2+ due to the chelation of the GSH ligands. The resulting aggregation amongst the GSH-GNPs in the presence of Pb2+ increases the aggregate size depending on the available Pb2+ ions, affecting the plasmonic coupling. This causes a substantial shift in the plasmon wavelength to a longer wavelength side with increasing Pb2+ concentration, resulting in a red-to-blue colorimetric or visual change, enabling the instant determination of lead content in water.
Keywords: calorimetric detection of metal ions; gold nanoparticles; surface plasmons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles.ACS Appl Mater Interfaces. 2010 May;2(5):1466-70. doi: 10.1021/am100107k. ACS Appl Mater Interfaces. 2010. PMID: 20429606
-
Highly sensitive colorimetric detection of lead using maleic acid functionalized gold nanoparticles.Talanta. 2015 Jan;132:613-8. doi: 10.1016/j.talanta.2014.10.024. Epub 2014 Oct 23. Talanta. 2015. PMID: 25476352
-
A biomimetic sensor for the detection of lead in water.Biosens Bioelectron. 2015 May 15;67:621-4. doi: 10.1016/j.bios.2014.09.077. Epub 2014 Oct 2. Biosens Bioelectron. 2015. PMID: 25449876
-
Identification of heavy metal ions from aqueous environment through gold, Silver and Copper Nanoparticles: An excellent colorimetric approach.Environ Res. 2022 Apr 1;205:112475. doi: 10.1016/j.envres.2021.112475. Epub 2021 Dec 1. Environ Res. 2022. PMID: 34863692 Review.
-
Colorimetric sensors for rapid detection of various analytes.Mater Sci Eng C Mater Biol Appl. 2017 Sep 1;78:1231-1245. doi: 10.1016/j.msec.2017.05.018. Epub 2017 May 5. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28575962 Review.
Cited by
-
Rapid visual dual-mode detection of Zr(IV) based on L-histidine functionalized gold nanoparticles.Anal Sci. 2024 Jul;40(7):1269-1278. doi: 10.1007/s44211-024-00557-z. Epub 2024 Apr 4. Anal Sci. 2024. PMID: 38575844
-
Colorimetric sensor for Pb2+ detection in water using thioglycolic acid-conjugated gold nanoparticles.RSC Adv. 2025 Mar 3;15(9):6931-6937. doi: 10.1039/d4ra06260d. eCollection 2025 Feb 26. RSC Adv. 2025. PMID: 40035007 Free PMC article.
References
-
- Advisory Committee on Childhood Lead Poisoning Prevention of the Centers for Disease Control and Prevention . Guidelines for Measuring Lead in Blood Using Point of Care Instruments. CDC; Atlanta, GA, USA: 2013. pp. 1–16.
-
- Lead Action . Lead Action News. The LEAD Group; Summer Hill, Australia: 1993.
-
- Anon Lead and Copper Rule, Drinking Water Requirements for States and Public Water Systems “US EPA”. [(accessed on 31 July 2023)];2008 Available online: https://www.epa.gov/dwreginfo/lead-and-copper-rule.
-
- United States Environmental Protection Agency Office of Water . Analytical Methods Approved for Drinking Water Compliance Monitoring of Inorganic Contaminants and Other Inorganic Constituents. EPA Publication; Washington, DC, USA: 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

