Rapid Quantification of Microvessels of Three-Dimensional Blood-Brain Barrier Model Using Optical Coherence Tomography and Deep Learning Algorithm
- PMID: 37622905
- PMCID: PMC10452445
- DOI: 10.3390/bios13080818
Rapid Quantification of Microvessels of Three-Dimensional Blood-Brain Barrier Model Using Optical Coherence Tomography and Deep Learning Algorithm
Abstract
The blood-brain barrier (BBB) is a selective barrier that controls the transport between the blood and neural tissue features and maintains brain homeostasis to protect the central nervous system (CNS). In vitro models can be useful to understand the role of the BBB in disease and assess the effects of drug delivery. Recently, we reported a 3D BBB model with perfusable microvasculature in a Transwell insert. It replicates several key features of the native BBB, as it showed size-selective permeability of different molecular weights of dextran, activity of the P-glycoprotein efflux pump, and functionality of receptor-mediated transcytosis (RMT), which is the most investigated pathway for the transportation of macromolecules through endothelial cells of the BBB. For quality control and permeability evaluation in commercial use, visualization and quantification of the 3D vascular lumen structures is absolutely crucial. Here, for the first time, we report a rapid, non-invasive optical coherence tomography (OCT)-based approach to quantify the microvessel network in the 3D in vitro BBB model. Briefly, we successfully obtained the 3D OCT images of the BBB model and further processed the images using three strategies: morphological imaging processing (MIP), random forest machine learning using the Trainable Weka Segmentation plugin (RF-TWS), and deep learning using pix2pix cGAN. The performance of these methods was evaluated by comparing their output images with manually selected ground truth images. It suggested that deep learning performed well on object identification of OCT images and its computation results of vessel counts and surface areas were close to the ground truth results. This study not only facilitates the permeability evaluation of the BBB model but also offers a rapid, non-invasive observational and quantitative approach for the increasing number of other 3D in vitro models.
Keywords: 3D BBB model; OCT image processing; vessel quantification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
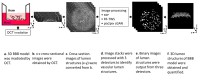


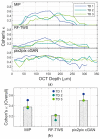

Similar articles
-
GCN-BBB: Deep Learning Blood-Brain Barrier (BBB) Permeability PharmacoAnalytics with Graph Convolutional Neural (GCN) Network.AAPS J. 2025 Apr 3;27(3):73. doi: 10.1208/s12248-025-01059-0. AAPS J. 2025. PMID: 40180695
-
Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1370-H1392. doi: 10.1152/ajpheart.00709.2020. Epub 2021 Feb 5. Am J Physiol Heart Circ Physiol. 2021. PMID: 33543687 Free PMC article.
-
Segmentation of the foveal microvasculature using deep learning networks.J Biomed Opt. 2016 Jul 1;21(7):75008. doi: 10.1117/1.JBO.21.7.075008. J Biomed Opt. 2016. PMID: 27401936
-
Machine Learning in Drug Development for Neurological Diseases: A Review of Blood Brain Barrier Permeability Prediction Models.Mol Inform. 2025 Mar;44(3):e202400325. doi: 10.1002/minf.202400325. Mol Inform. 2025. PMID: 40146590 Free PMC article. Review.
-
The Isolated Brain Microvessel: A Versatile Experimental Model of the Blood-Brain Barrier.Front Physiol. 2020 May 7;11:398. doi: 10.3389/fphys.2020.00398. eCollection 2020. Front Physiol. 2020. PMID: 32457645 Free PMC article. Review.
Cited by
-
Optical coherence tomography (OCT) and OCT angiography: Technological development and applications in brain science.Theranostics. 2025 Jan 1;15(1):122-140. doi: 10.7150/thno.97192. eCollection 2025. Theranostics. 2025. PMID: 39744229 Free PMC article. Review.
-
Updates on cancer vaccines in brain cancer: Advances in neuroblastoma, delivery systems, and emerging technologies.Hum Vaccin Immunother. 2025 Dec;21(1):2526964. doi: 10.1080/21645515.2025.2526964. Epub 2025 Jul 8. Hum Vaccin Immunother. 2025. PMID: 40627495 Free PMC article. Review.
References
-
- Qi D., Wu S., Lin H., Kuss M.A., Lei Y., Krasnoslobodtsev A., Ahmed S., Zhang C., Kim H.J., Jiang P., et al. Establishment of a Human IPSC- and Nanofiber-Based Microphysiological Blood–Brain Barrier System. ACS Appl. Mater. Interfaces. 2018;10:21825–21835. doi: 10.1021/acsami.8b03962. - DOI - PMC - PubMed
-
- Nzou G., Wicks R.T., Wicks E.E., Seale S.A., Sane C.H., Chen A., Murphy S.V., Jackson J.D., Atala A.J. Human Cortex Spheroid with a Functional Blood Brain Barrier for High-Throughput Neurotoxicity Screening and Disease Modeling. Sci. Rep. 2018;8:7413. doi: 10.1038/s41598-018-25603-5. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

