Coupling Capillary-Driven Microfluidics with Lateral Flow Immunoassay for Signal Enhancement
- PMID: 37622918
- PMCID: PMC10452194
- DOI: 10.3390/bios13080832
Coupling Capillary-Driven Microfluidics with Lateral Flow Immunoassay for Signal Enhancement
Abstract
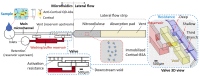
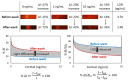
Microfluidics has emerged as a versatile technology that is applied to enhance the performance of analytical techniques, among others. Pursuing this, we present a capillary-driven microfluidic device that improves the sensitivity of lateral flow immunoassay rapid tests thanks to offering an automated washing step. A novel multilevel microfluidic chip was 3D-printed with a photocurable black resin, sealed by an optically clear pressure-sensitive adhesive, and linked to the lateral flow strip. To depict the efficacy of microfluidics and the washing step, cortisol was measured quantitatively within the proposed device. Measuring cortisol levels is a way to capture physiological stress responses. Among biofluids, saliva is less infectious and easier to sample than others. However, higher sensitivity is demanded because the salivary cortisol concentrations are much lower than in blood. We carried out a competitive lateral flow immunoassay protocol with the difference that the microfluidic device applies an automated washing step after the sample is drained downstream. It washes the trapped quantum-dot-labeled antibodies out from nitrocellulose, diminishing background noise as these are bonded to cortisols and not to the immobilized receptors. Fluorescence spectroscopy, as a high-precision analysis, was successfully applied to determine clinically relevant salivary cortisol concentrations within a buffer quantitatively. The microfluidic design relied on a 3D valve that avoids reagent cross-contamination. This cross-contamination could make the washing buffer impure and undesirably dilute the sample. The proposed device is cost-effective, self-powered, robust, and ideal for non-expert users.
Keywords: 3D-printing; capillary valve; capillary-driven microfluidics; cortisol; fluorescence spectroscopy; lateral flow assay.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates.Lab Chip. 2009 Dec 7;9(23):3330-7. doi: 10.1039/b906523g. Epub 2009 Aug 21. Lab Chip. 2009. PMID: 19904397
-
Diffusion-free valve for preprogrammed immunoassay with capillary microfluidics.Microsyst Nanoeng. 2023 Jul 17;9:91. doi: 10.1038/s41378-023-00568-2. eCollection 2023. Microsyst Nanoeng. 2023. PMID: 37469685 Free PMC article.
-
Improving design features and air bubble manipulation techniques for a single-step sandwich electrochemical ELISA incorporating commercial electrodes into capillary-flow driven immunoassay devices.Analyst. 2024 Mar 25;149(7):2034-2044. doi: 10.1039/d3an01704d. Analyst. 2024. PMID: 38407468 Free PMC article.
-
Application of Microfluidics in Immunoassay: Recent Advancements.J Healthc Eng. 2021 Jul 15;2021:2959843. doi: 10.1155/2021/2959843. eCollection 2021. J Healthc Eng. 2021. PMID: 34326976 Free PMC article. Review.
-
A survey of 3D printing technology applied to paper microfluidics.Lab Chip. 2021 Dec 21;22(1):9-25. doi: 10.1039/d1lc00768h. Lab Chip. 2021. PMID: 34897346 Review.
Cited by
-
Development of a syringe-hosted load-and-read immunoassay device using autoinjected distance readout in paper inserts.Mikrochim Acta. 2025 Feb 28;192(3):195. doi: 10.1007/s00604-025-07052-w. Mikrochim Acta. 2025. PMID: 40016539
-
Rapid prototyping of thermoplastic microfluidic devices via SLA 3D printing.Sci Rep. 2024 Jul 31;14(1):17646. doi: 10.1038/s41598-024-68761-5. Sci Rep. 2024. PMID: 39085631 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

