Cholera intoxication of human enteroids reveals interplay between decoy and functional glycoconjugate ligands
- PMID: 37622990
- PMCID: PMC10629719
- DOI: 10.1093/glycob/cwad069
Cholera intoxication of human enteroids reveals interplay between decoy and functional glycoconjugate ligands
Abstract
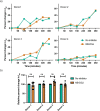
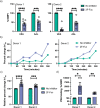
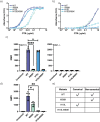
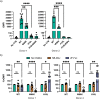
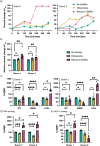
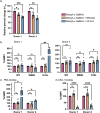
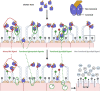
Prior research on cholera toxin (CT) binding and intoxication has relied on human colonic cancer derived epithelial cells. While these transformed cell lines have been beneficial, they neither derive from small intestine where intoxication occurs, nor represent the diversity of small intestinal epithelial cells (SI-ECs) and variation in glycoconjugate expression among individuals. Here, we used human enteroids, derived from jejunal biopsies of multipledonors to study CT binding and intoxication of human non-transformed SI-ECs. We modulated surface expression of glycosphingolipids, glycoproteins and specific glycans to distinguish the role of each glycan/glycoconjugate. Cholera-toxin-subunit-B (CTB) mutants were generated to decipher the preference of each glycoconjugate to different binding sites and the correlation between CT binding and intoxication. Human enteroids contain trace amounts of GM1, but other glycosphingolipids may be contributing to CT intoxication. We discovered that inhibition of either fucosylation or O-glycosylation sensitize enteroids to CT-intoxication. This can either be a consequence of the removal of fucosylated "decoy-like-ligands" binding to CTB's non-canonical site and/or increase in the availability of Gal/GalNAc-terminating glycoconjugates binding to the canonical site. Furthermore, simultaneous inhibition of fucosylation and O-glycosylation increased the availability of additional Gal/GalNAc-terminating glycoconjugates but counteracted the sensitization in CT intoxication caused by inhibiting O-glycosylation because of reduction in fucose. This implies a dual role of fucose as a functional glycan and a decoy, the interplay of which influences CT binding and intoxication. Finally, while the results were similar for enteroids from different donors, they were not identical, pointing to a role for human genetic variation in determining sensitivity to CT.
Keywords: O-glycosylation; cholera toxin; decoy like ligands; enteroid monolayers; fucosylation.
© The Author(s) 2023. Published by Oxford University Press.
Figures
Similar articles
-
Fucosylation of glycoproteins and glycolipids: opposing roles in cholera intoxication.Nat Chem Biol. 2025 Apr;21(4):555-566. doi: 10.1038/s41589-024-01748-5. Epub 2024 Oct 16. Nat Chem Biol. 2025. PMID: 39414978 Free PMC article.
-
Fucose-Galactose Polymers Inhibit Cholera Toxin Binding to Fucosylated Structures and Galactose-Dependent Intoxication of Human Enteroids.ACS Infect Dis. 2020 May 8;6(5):1192-1203. doi: 10.1021/acsinfecdis.0c00009. Epub 2020 Mar 19. ACS Infect Dis. 2020. PMID: 32134631 Free PMC article.
-
Fucosylated Molecules Competitively Interfere with Cholera Toxin Binding to Host Cells.ACS Infect Dis. 2018 May 11;4(5):758-770. doi: 10.1021/acsinfecdis.7b00085. Epub 2018 Feb 22. ACS Infect Dis. 2018. PMID: 29411974 Free PMC article.
-
Therapeutic Potential of Cholera Toxin B Subunit for the Treatment of Inflammatory Diseases of the Mucosa.Toxins (Basel). 2017 Nov 23;9(12):379. doi: 10.3390/toxins9120379. Toxins (Basel). 2017. PMID: 29168738 Free PMC article. Review.
-
Cholera: pathophysiology and emerging therapeutic targets.Future Med Chem. 2013 May;5(7):781-98. doi: 10.4155/fmc.13.42. Future Med Chem. 2013. PMID: 23651092 Review.
Cited by
-
Hemagglutinin Protease HapA Associated With Vibrio cholerae Outer Membrane Vesicles (OMVs) Disrupts Tight and Adherens Junctions.J Extracell Vesicles. 2025 May;14(5):e70092. doi: 10.1002/jev2.70092. J Extracell Vesicles. 2025. PMID: 40415227 Free PMC article.
-
Fucosylation of glycoproteins and glycolipids: opposing roles in cholera intoxication.Nat Chem Biol. 2025 Apr;21(4):555-566. doi: 10.1038/s41589-024-01748-5. Epub 2024 Oct 16. Nat Chem Biol. 2025. PMID: 39414978 Free PMC article.
-
Sortase-Modified Cholera Toxoids Show Specific Golgi Localization.Toxins (Basel). 2024 Apr 16;16(4):194. doi: 10.3390/toxins16040194. Toxins (Basel). 2024. PMID: 38668619 Free PMC article.
-
Fucosylated glycoproteins and fucosylated glycolipids play opposing roles in cholera intoxication.bioRxiv [Preprint]. 2023 Aug 3:2023.08.02.551727. doi: 10.1101/2023.08.02.551727. bioRxiv. 2023. Update in: Nat Chem Biol. 2025 Apr;21(4):555-566. doi: 10.1038/s41589-024-01748-5. PMID: 37577488 Free PMC article. Updated. Preprint.
-
The Mutagenic Plasticity of the Cholera Toxin B-Subunit Surface Residues: Stability and Affinity.Toxins (Basel). 2024 Mar 4;16(3):133. doi: 10.3390/toxins16030133. Toxins (Basel). 2024. PMID: 38535799 Free PMC article.
References
-
- Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman JA, Shukla GS, Radin NS. Improved inhibitors of glucosylceramide synthase. J Biochem (Tokyo). 1992:111(2):191–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

