β-Caryophyllene-Loaded Microemulsion-Based Topical Hydrogel: A Promising Carrier to Enhance the Analgesic and Anti-Inflammatory Outcomes
- PMID: 37623089
- PMCID: PMC10454053
- DOI: 10.3390/gels9080634
β-Caryophyllene-Loaded Microemulsion-Based Topical Hydrogel: A Promising Carrier to Enhance the Analgesic and Anti-Inflammatory Outcomes
Abstract
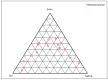
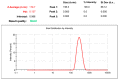
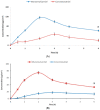
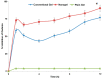
Musculoskeletal pain and inflammation can vary from localised pain like pain in the shoulders and neck to widespread pain like fibromyalgia, and as per estimates, around 90% of humans have experienced such pain. Oral non-steroidal anti-inflammatory drugs (NSAIDs) are frequently prescribed for such conditions but are associated with concerns like gastric irritation and bleeding. In the present study, a microemulsion-based gel comprising β-caryophyllene, isopropyl myristate, Tween 80, and normal saline was prepared as a topical option for managing topical pain and inflammation. The globules of the microemulsion were below 100 nm with a zetapotential of around -10 mV. The drug entrapment was >87% with a drug loading of >23%. The permeation studies established better skin permeation (20.11 ± 0.96 μg cm-2 h-1) and retention of the drug (4.96 ± 0.02%) from the developed system vis-à-vis the conventional product (9.73 ± 0.35 μg cm-2 h-1; 1.03 ± 0.01%). The dermatokinetic studies established the better pharmacokinetic profile of the bioactive in the epidermis and dermis layers of the skin. The anti-inflammatory potential in carrageenan-induced rat paw oedema was more pronounced than the conventional product (~91% vis-à-vis ~77%), indicating a better pharmacodynamic outcome from the developed system. The nanotechnology-based natural bioactive product with improved efficacy and drug loading can provide a better alternative for the management of musculoskeletal pain.
Keywords: analgesia; bioavailability; dermatokinetics; microemulsion; nanocarrier; safety; skin permeation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Benzyl Benzoate-Loaded Microemulsion for Topical Applications: Enhanced Dermatokinetic Profile and Better Delivery Promises.AAPS PharmSciTech. 2016 Oct;17(5):1221-31. doi: 10.1208/s12249-015-0464-0. Epub 2015 Dec 15. AAPS PharmSciTech. 2016. PMID: 26669889
-
Microemulsion-Based Keratin-Chitosan Gel for Improvement of Skin Permeation/Retention and Activity of Curcumin.Gels. 2023 Jul 21;9(7):587. doi: 10.3390/gels9070587. Gels. 2023. PMID: 37504466 Free PMC article.
-
Superiority of microemulsion-based hydrogel for non-steroidal anti-inflammatory drug transdermal delivery: a comparative safety and anti-nociceptive efficacy study.Int J Pharm. 2022 Jun 25;622:121830. doi: 10.1016/j.ijpharm.2022.121830. Epub 2022 May 16. Int J Pharm. 2022. PMID: 35589005
-
Implementing Nanovesicles for Boosting the Skin Permeation of Non-steroidal Anti-inflammatory Drugs.AAPS PharmSciTech. 2023 Sep 28;24(7):195. doi: 10.1208/s12249-023-02649-x. AAPS PharmSciTech. 2023. PMID: 37770750 Review.
-
Topical NSAID therapy for musculoskeletal pain.Pain Med. 2010 Apr;11(4):535-49. doi: 10.1111/j.1526-4637.2010.00809.x. Epub 2010 Mar 4. Pain Med. 2010. PMID: 20210866 Review.
References
-
- Suhail N., Alzahrani A.K., Basha W.J., Kizilbash N., Zaidi A., Ambreen J., Khachfe H.M. Microemulsions: Unique properties, pharmacological applications, and targeted drug delivery. Front. Nanotechnol. 2021;3:754889. doi: 10.3389/fnano.2021.754889. - DOI
LinkOut - more resources
Full Text Sources

