The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model
- PMID: 37623223
- PMCID: PMC10453891
- DOI: 10.3390/cimb45080404
The Effect of the Mixed Extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai on the Improvement of Degenerative Osteoarthritis through Inflammation Inhibition in the Monosodium Iodoacetate-Induced Mouse Model
Abstract
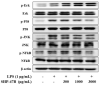
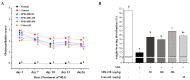
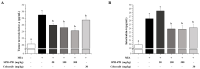
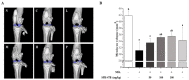
Osteoarthritis is a chronic inflammatory disease, and, due to the lack of fundamental treatment, the main objective is to alleviate pain and prevent cartilage damage. Kalopanax pictus Nakai and Achyranthes japonica Nakai are herbal plants known for their excellent anti-inflammatory properties. The objective of this study is to confirm the potential of a mixture extract of Kalopanax pictus Nakai and Achyranthes japonica Nakai as a functional raw material for improving osteoarthritis through anti-inflammatory effects in macrophages and MIA-induced arthritis experimental animals. In macrophages inflamed by lipopolysaccharide (LPS), treatment of Kalopanax pictus Nakai and Achyranthes japonica Nakai mixture inhibits NF-κB and mitogen-activated protein kinase (MAPK) activities, thereby inhibiting inflammatory cytokine tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6), inflammatory factors PGE2, MMP-2, and MMP-9, and nitric oxide (NO) was reduced. In addition, in an animal model of arthritis induced by MIA (monosodium iodoacetate), administration of Kalopanax pictus Nakai and Achyranthes japonica Nakai mixture reduced blood levels of inflammatory cytokines TNF-α and IL-6, inflammatory factors prostaglandin E2(PGE2), matrix metalloproteinase-2(MMP-2), and NO. Through these anti-inflammatory effects, MIA-induced pain reduction (recovery of clinical index, increase in weight bearing, and increase in area and width of the foot), recovery of meniscus damage, loss of cartilage tissue or inflammatory cells in tissue infiltration reduction, and recovery of the proteglycan layer were confirmed. Therefore, it is considered that Kalopanax pictus Nakai and Achyranthes japonica Nakai mixture has the potential as a functional raw material that promotes joint health.
Keywords: Achyranthes japonica Nakai; Kalopanax pictus Nakai; MMPs; anti-inflammation; osteoarthritis.
Conflict of interest statement
Authors Y.M.P., H.Y.L., D.Y.S., H.Y.P., H.M.H., H.N.J. and J.G.K. were employed by the company INVIVO Co., Ltd. Authors S.H.K. and M.J.K. were employed by the Agricultural Corporation Company Nongjeongsim LC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Nielsen L.A., Schepman P., Blakeman K.H., Wilhelm S., Robinson R., Beck C., Hansen J.L., Rolfson O. Prescription patterns and predictors of unmet pain relief in patients with difficult-to-treat osteoarthritis in the Nordics: Analyses from the BISCUITS study. Scand. J. Pain. 2022;23:149–160. doi: 10.1515/sjpain-2021-0211. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

