Precision Medicine in Erythropoietin Deficiency and Treatment Resistance: A Novel Approach to Management of Anaemia in Chronic Kidney Disease
- PMID: 37623232
- PMCID: PMC10453742
- DOI: 10.3390/cimb45080413
Precision Medicine in Erythropoietin Deficiency and Treatment Resistance: A Novel Approach to Management of Anaemia in Chronic Kidney Disease
Abstract
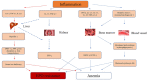
The study of anaemia is a well-developed discipline where the concepts of precision medicine have, in part, been researched extensively. This review discusses the treatment of erythropoietin (EPO) deficiency anaemia and resistance in cases of chronic kidney disease (CKD). Traditionally, erythropoietin-stimulating agents (ESAs) and iron supplementation have been used to manage anaemia in cases of CKD. However, these treatments pose potential risks, including cardiovascular and thromboembolic events. Newer treatments have emerged to address these risks, such as slow-release and low-dosage intravenous iron, oral iron supplementation, and erythropoietin-iron combination therapy. Another novel approach is the use of hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs). This review highlights the need for precision medicine targeting the genetic components of EPO deficiency anaemia in CKD and discusses individual variability in genes such as the erythropoietin gene (EPO), the interleukin-β gene (IL-β), and the hypoxia-inducible factor gene (HIF). Pharmacogenetic testing aims to provide targeted therapies and interventions that are tailored to the specific characteristics of an individual, thus optimising treatment outcomes and minimising resistance and adverse effects. This article concludes by suggesting that receptor modification has the potential to revolutionise the treatment outcomes of patients with erythropoietin deficiency anaemia through the integration of the mentioned approach.
Keywords: HIF-PHI; erythropoietin deficiency anaemia; inflammation; pharmacogenetics; precision medicine.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Fishbane S., El-Shahawy M.A., Pecoits-Filho R., Pham Van B., Houser M.T., Frison L., Little D.J., Guzman N.J., Pergola P.E. OLYMPUS: A Phase 3, Randomized, Double-Blind, Placebo-Controlled, International Study of Roxadustat Efficacy in Patients with Non-Dialysis-Dependent (NDD) CKD and Anemia [Abstract TH-OR023] J. Am. Soc. Nephrol. 2019;30:6.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

