Advances in S. cerevisiae Engineering for Xylose Fermentation and Biofuel Production: Balancing Growth, Metabolism, and Defense
- PMID: 37623557
- PMCID: PMC10455348
- DOI: 10.3390/jof9080786
Advances in S. cerevisiae Engineering for Xylose Fermentation and Biofuel Production: Balancing Growth, Metabolism, and Defense
Abstract
Genetically engineering microorganisms to produce chemicals has changed the industrialized world. The budding yeast Saccharomyces cerevisiae is frequently used in industry due to its genetic tractability and unique metabolic capabilities. S. cerevisiae has been engineered to produce novel compounds from diverse sugars found in lignocellulosic biomass, including pentose sugars, like xylose, not recognized by the organism. Engineering high flux toward novel compounds has proved to be more challenging than anticipated since simply introducing pathway components is often not enough. Several studies show that the rewiring of upstream signaling is required to direct products toward pathways of interest, but doing so can diminish stress tolerance, which is important in industrial conditions. As an example of these challenges, we reviewed S. cerevisiae engineering efforts, enabling anaerobic xylose fermentation as a model system and showcasing the regulatory interplay's controlling growth, metabolism, and stress defense. Enabling xylose fermentation in S. cerevisiae requires the introduction of several key metabolic enzymes but also regulatory rewiring of three signaling pathways at the intersection of the growth and stress defense responses: the RAS/PKA, Snf1, and high osmolarity glycerol (HOG) pathways. The current studies reviewed here suggest the modulation of global signaling pathways should be adopted into biorefinery microbial engineering pipelines to increase efficient product yields.
Keywords: environmental stress response; protein kinase A; signal transduction; xylose fermentation; yeast.
Conflict of interest statement
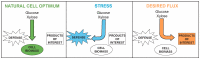
Our understanding of how growth, metabolism, and defense are integrated into cellular regulatory networks is only beginning to emerge, but recent studies have uncovered new insights into the balance between the growth and defense controls. Rapid growth and maximal stress tolerance are competing interests in the cell since both require significant resources to enact. When times are good and nutrients are plentiful,
A major component of the
Activation of the ESR can co-occur with the decreased growth rate of a culture, leading several studies to suggest that the ESR is intimately regulated with, and predictive of, the cellular growth rate [30,44,45,46]. However, work from our lab shows that the ESR is separable from growth and division: the ESR is still activated upon heat or salt stress, even in cells that are already arrested in their cell cycle with low biomass production [34]. Instead, we argue that the dramatic transcriptome changes associated with the ESR serve to accelerate a stress response. The transient repression of ribosome-related and growth-promoting genes during stress helps to redirect the transcriptional and translational capacity toward stress-induced transcripts [34,47,48]. Somewhat counterintuitively, cells that lack repressors of the repressed ESR genes, Dot6 and Tod6, grow well in the absence of stress but acclimate much slower to salt stress [48]. At least part of this mutant effect can be explained by the delayed production of defense proteins: ribosome- and growth-related transcripts stay associated with translating ribosomes in the mutant cells at the expense of stress-induced transcripts, leading to a delay in the production of stress defense proteins [34,48].
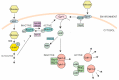
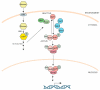
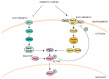
The ESR is regulated by multiple upstream signaling pathways, many of which are only activated by specific conditions [33,34,37,38]. Among the best studied of these are the protein kinase A (PKA), Snf1, and high osmolarity glycerol (HOG) pathways, all of which turn out to be important for engineered xylose fermentation (reviewed in more detail below). PKA inhibits the ESR in part by phosphorylating and inhibiting Msn2/4, Dot6/Tod6, and other regulators [49,50]; it also functions to modulate gene expression of specific genes by binding promoters and coding regions via interactions with chromatin proteins or the RNA polymerase [51,52,53,54]. Snf1 and HOG can also modulate downstream ESR regulators, including Msn2/4 and others, both directly and indirectly [41,55,56,57]. Interestingly, several independent studies found that modulating the activity of these broadly acting signaling pathways is necessary to promote robust anaerobic fermentation of xylose in engineered biofuel yeast strains (see below). The remainder of this review will discuss the potential roles of the PKA, Snf1, and HOG pathways in engineering xylose fermentation.
The authors declare no conflict of interest.
Figures
Similar articles
-
Rewired cellular signaling coordinates sugar and hypoxic responses for anaerobic xylose fermentation in yeast.PLoS Genet. 2019 Mar 11;15(3):e1008037. doi: 10.1371/journal.pgen.1008037. eCollection 2019 Mar. PLoS Genet. 2019. PMID: 30856163 Free PMC article.
-
PKA and HOG signaling contribute separable roles to anaerobic xylose fermentation in yeast engineered for biofuel production.PLoS One. 2019 May 21;14(5):e0212389. doi: 10.1371/journal.pone.0212389. eCollection 2019. PLoS One. 2019. PMID: 31112537 Free PMC article.
-
Xylose Assimilation for the Efficient Production of Biofuels and Chemicals by Engineered Saccharomyces cerevisiae.Biotechnol J. 2021 Apr;16(4):e2000142. doi: 10.1002/biot.202000142. Epub 2020 Nov 13. Biotechnol J. 2021. PMID: 33135317 Review.
-
Genome-scale consequences of cofactor balancing in engineered pentose utilization pathways in Saccharomyces cerevisiae.PLoS One. 2011;6(11):e27316. doi: 10.1371/journal.pone.0027316. Epub 2011 Nov 4. PLoS One. 2011. PMID: 22076150 Free PMC article.
-
Value-added biotransformation of cellulosic sugars by engineered Saccharomyces cerevisiae.Bioresour Technol. 2018 Jul;260:380-394. doi: 10.1016/j.biortech.2018.04.013. Epub 2018 Apr 7. Bioresour Technol. 2018. PMID: 29655899 Review.
Cited by
-
Optimization of the enrichment medium for recombinant ChIL-4-ChIL-2 in Lactococcus lactis through response surface methodology.Poult Sci. 2025 May;104(5):105025. doi: 10.1016/j.psj.2025.105025. Epub 2025 Mar 14. Poult Sci. 2025. PMID: 40120242 Free PMC article.
References
-
- Cravens A., Payne J., Smolke C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. [(accessed on 14 February 2023)];Nat. Commun. 2019 10:2142. doi: 10.1038/s41467-019-09848-w. Available online: https://www.nature.com/articles/s41467-019-09848-w. - DOI - PMC - PubMed
-
- Money N.P. The Rise of Yeast: How the Sugar Fungus Shaped Civilization. 2018. [(accessed on 17 February 2023)]. p. 210. Available online: https://global.oup.com/academic/product/the-rise-of-yeast-9780198749707.
-
- Samuel D. Investigation of Ancient Egyptian Baking and Brewing Methods by Correlative Microscopy. [(accessed on 17 February 2023)];Science. 1996 273:488–490. doi: 10.1126/science.273.5274.488. Available online: https://pubmed.ncbi.nlm.nih.gov/8662535/ - DOI - PubMed
-
- Nielsen J. Yeast Systems Biology: Model Organism and Cell Factory. [(accessed on 14 February 2023)];Biotechnol. J. 2019 14:1800421. doi: 10.1002/biot.201800421. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/biot.201800421. - DOI - DOI - PubMed
-
- Schindler D. Genetic Engineering and Synthetic Genomics in Yeast to Understand Life and Boost Biotechnology. [(accessed on 14 February 2023)];Bioengineering. 2020 7:137. doi: 10.3390/bioengineering7040137. Available online: https://www.mdpi.com/2306-5354/7/4/137/htm. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

