The Role of Lignin in the Compartmentalization of Cadmium in Maize Roots Is Enhanced by Mycorrhiza
- PMID: 37623623
- PMCID: PMC10455880
- DOI: 10.3390/jof9080852
The Role of Lignin in the Compartmentalization of Cadmium in Maize Roots Is Enhanced by Mycorrhiza
Abstract
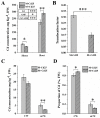
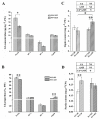
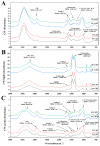
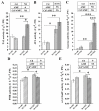
In nature, arbuscular mycorrhizal fungi (AMF) play a crucial role in the root systems of plants. They can help enhance the resistance of host plants by improving the compartmentalization of toxic metal contaminants in the cell walls (CWs). However, the functions and responses of various CW subfractions to mycorrhizal colonization under Cd exposure remain unknown. Here we conducted a study to investigate how Cd is stored in the cell walls of maize roots colonized by Funneliformis mosseae. Our findings indicate that inoculating the roots with AMF significantly lowers the amount of Cd in the maize shoots (63.6 ± 6.54 mg kg-1 vs. 45.3 ± 2.19 mg kg-1, p < 0.05) by retaining more Cd in the mycorrhized roots (224.0 ± 17.13 mg kg-1 vs. 289.5 ± 8.75 mg kg-1, p < 0.01). This reduces the adverse effects of excessive Cd on the maize plant. Additional research on the subcellular distribution of Cd showed that AMF colonization significantly improves the compartmentalization of 88.2% of Cd in the cell walls of maize roots, compared to the 80.8% of Cd associated with cell walls in the non-mycorrhizal controls. We observed that the presence of AMF did not increase the amount of Cd in pectin, a primary binding site for cell walls; however, it significantly enhanced the content of lignin and the proportion of Cd in the total root cell walls. This finding is consistent with the increased activity of lignin-related enzymes, such as PAL, 4CL, and laccase, which were also positively impacted by mycorrhizal colonization. Fourier transform infrared spectroscopy (FTIR) results revealed that AMF increased the number and types of functional groups, including -OH/-NH and carboxylate, which chelate Cd in the lignin. Our research shows that AMF can improve the ability of maize plants to tolerate Cd by reducing the amount of Cd transferred from the roots to the shoots. This is achieved by increasing the amount of lignin in the cell walls, which binds with Cd and prevents it from moving through the plant. This is accomplished by activating enzymes related to lignin synthesis and increasing the exposure of Cd-binding functional groups of lignin. However, more direct evidence on the immobilization of Cd in the mycorrhiza-altered cell wall subfractions is needed.
Keywords: Cd tolerance; arbuscular mycorrhizal fungus (AMF); cell walls (CWs); lignin; maize (Zea mays).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
An arbuscular mycorrhizal fungus differentially regulates root traits and cadmium uptake in two maize varieties.Ecotoxicol Environ Saf. 2023 Oct 1;264:115458. doi: 10.1016/j.ecoenv.2023.115458. Epub 2023 Sep 8. Ecotoxicol Environ Saf. 2023. PMID: 37690173
-
Cadmium transfer between maize and soybean plants via common mycorrhizal networks.Ecotoxicol Environ Saf. 2022 Mar 1;232:113273. doi: 10.1016/j.ecoenv.2022.113273. Epub 2022 Feb 3. Ecotoxicol Environ Saf. 2022. PMID: 35123184
-
Effect of an arbuscular mycorrhizal fungus on maize growth and cadmium migration in a sand column.Ecotoxicol Environ Saf. 2021 Dec 1;225:112782. doi: 10.1016/j.ecoenv.2021.112782. Epub 2021 Sep 15. Ecotoxicol Environ Saf. 2021. PMID: 34536792
-
Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays.Ecotoxicol Environ Saf. 2019 Apr 30;171:352-360. doi: 10.1016/j.ecoenv.2018.12.097. Epub 2019 Jan 4. Ecotoxicol Environ Saf. 2019. PMID: 30616152
-
Arbuscular Mycorrhizal Fungi-Assisted Phytoremediation: A Promising Strategy for Cadmium-Contaminated Soils.Plants (Basel). 2024 Nov 22;13(23):3289. doi: 10.3390/plants13233289. Plants (Basel). 2024. PMID: 39683082 Free PMC article. Review.
Cited by
-
Research progress of the detection and analysis methods of heavy metals in plants.Front Plant Sci. 2024 Jan 31;15:1310328. doi: 10.3389/fpls.2024.1310328. eCollection 2024. Front Plant Sci. 2024. PMID: 38362447 Free PMC article. Review.
-
Uncovering differences in cadmium accumulation capacity of different Ipomoea aquatica cultivars at the level of root cell types.Hortic Res. 2025 Mar 11;12(6):uhaf077. doi: 10.1093/hr/uhaf077. eCollection 2025 Jun. Hortic Res. 2025. PMID: 40297020 Free PMC article.
-
Bioremediation of Contaminated Soil by Fungi: A Call for Research.J Fungi (Basel). 2024 Sep 29;10(10):684. doi: 10.3390/jof10100684. J Fungi (Basel). 2024. PMID: 39452636 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

