Development of Nanosuspension of Artemisia absinthium Extract as Novel Drug Delivery System to Enhance Its Bioavailability and Hepatoprotective Potential
- PMID: 37623677
- PMCID: PMC10456093
- DOI: 10.3390/jfb14080433
Development of Nanosuspension of Artemisia absinthium Extract as Novel Drug Delivery System to Enhance Its Bioavailability and Hepatoprotective Potential
Abstract
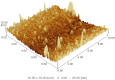
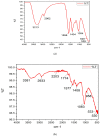
A nanosuspension of Artemisia absinthium extract was formulated and characterized for the enhancement of bioavailability and better hepatoprotective efficacy. The nanosuspension of A. absinthium extract was formulated using an antisolvent precipitation technique, and various formulation parameters were optimized using response surface methodology (RSM). The optimized nanosuspension was characterized using AFM and FT-IR spectroscopy. The drug-release profile and oral bioavailability of the optimized nanosuspension were assessed with reference to coarse suspension. The DPPH radical scavenging method was used to measure the nanosuspension's antioxidant activity, and its in vivo hepatoprotective potential was assessed against CCl4-induced hepatic injury in rats. The developed optimized nanosuspension had suitable zeta potential of -11.9 mV, PDI of 0.285, and mean particle size of 253.8 nm. AFM study demonstrated a homogeneous population of nanoparticles with average size of 25 nm. The formulated nanosuspension of A. absinthium showed faster dissolution rate and 1.13-fold enhanced bioavailability as compared to the coarse suspension (plant extract). Furthermore, the nanoformulation had stronger antioxidant and hepatoprotective potential as compared to the unprocessed coarse extract. These results demonstrated that nanosuspension is a promising strategy for improving the oral bioavailability and bioactivities of A. absinthium extract.
Keywords: Artemisia absinthium; bioavailability; nanosuspension; response surface methodology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Increased Oral Bioavailability of Piperine from an Optimized Piper nigrum Nanosuspension.Planta Med. 2019 Feb;85(3):249-257. doi: 10.1055/a-0759-2208. Epub 2018 Oct 24. Planta Med. 2019. PMID: 30357764
-
Exploring the Solvent-Anti-solvent Method of Nanosuspension for Enhanced Oral Bioavailability of Lovastatin.Turk J Pharm Sci. 2021 Oct 28;18(5):541-549. doi: 10.4274/tjps.galenos.2020.65047. Turk J Pharm Sci. 2021. PMID: 34708645 Free PMC article.
-
Nanoformulation of Curcuma longa Root Extract and Evaluation of Its Dissolution Potential.ACS Omega. 2022 Dec 23;8(1):1088-1096. doi: 10.1021/acsomega.2c06258. eCollection 2023 Jan 10. ACS Omega. 2022. PMID: 36643543 Free PMC article.
-
Nasal delivery of nanosuspension-based mucoadhesive formulation with improved bioavailability of loratadine: Preparation, characterization, and in vivo evaluation.Int J Pharm. 2020 Apr 15;579:119166. doi: 10.1016/j.ijpharm.2020.119166. Epub 2020 Feb 19. Int J Pharm. 2020. PMID: 32084574
-
An overview of recent patents on nanosuspension.Recent Pat Drug Deliv Formul. 2014;8(2):144-54. doi: 10.2174/1872211308666140422150819. Recent Pat Drug Deliv Formul. 2014. PMID: 24758487 Review.
Cited by
-
Improving the Anti-Tumor Effect of Indoleamine 2,3-Dioxygenase Inhibitor CY1-4 by CY1-4 Nano-Skeleton Drug Delivery System.J Funct Biomater. 2024 Dec 9;15(12):372. doi: 10.3390/jfb15120372. J Funct Biomater. 2024. PMID: 39728172 Free PMC article.
-
Advanced Strategies in Enhancing the Hepatoprotective Efficacy of Natural Products: Integrating Nanotechnology, Genomics, and Mechanistic Insights.ACS Biomater Sci Eng. 2025 May 12;11(5):2528-2549. doi: 10.1021/acsbiomaterials.5c00004. Epub 2025 Apr 11. ACS Biomater Sci Eng. 2025. PMID: 40211874 Free PMC article. Review.
References
-
- Ananth D.A., Tietela Z., Aseervatham G.S.B., Garlapatic D., Sivasudhad T. Phytochemical and pharmacological status of indigenous medicinal plant Pedalium murex L.—A review. Biomed. Pharmacother. 2018;103:1456–1463. - PubMed
-
- Tlili N., Tir M., Feriani A., Yahia Y., Allagui M.S., Saadaoui E., Cafsi M.E., Nasri N. Potential health advantages of Periploca laevigata: Preliminary phytochemical analysis and evaluation of in vitro antioxidant capacity and assessment of hepatoprotective, anti-inflammatory and analgesic effects. J. Funct. Foods. 2018;48:234–242. doi: 10.1016/j.jff.2018.06.028. - DOI
-
- Junejo J.A., Gogoi G., Islam J., Rudrapal M., Mondal P., Hazarika H., Zaman K. Exploration of antioxidant, antidiabetic and hepatoprotective activity of Diplazium esculentum—A wild edible plant from North Eastern India. Future J. Pharm. Sci. 2018;4:93–101. doi: 10.1016/j.fjps.2017.10.005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

