Selective Optical Imaging for Detection of Bacterial Biofilms in Tissues
- PMID: 37623692
- PMCID: PMC10455256
- DOI: 10.3390/jimaging9080160
Selective Optical Imaging for Detection of Bacterial Biofilms in Tissues
Abstract
Significance: The development of an imaging technique to accurately identify biofilm regions on tissues and in wounds is crucial for the implementation of precise surface-based treatments, leading to better patient outcomes and reduced chances of infection.
Aim: The goal of this study was to develop an imaging technique that relies on selective trypan blue (TB) staining of dead cells, necrotic tissues, and bacterial biofilms, to identify biofilm regions on tissues and wounds.
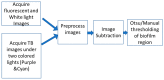
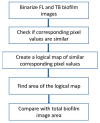
Approach: The study explored combinations of ambient multi-colored LED lights to obtain maximum differentiation between stained biofilm regions and the underlying chicken tissue or glass substrate during image acquisition. The TB imaging results were then visually and statistically compared to fluorescence images using a shape similarity measure.
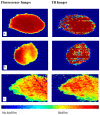
Results: The comparisons between the proposed TB staining method and the fluorescence standard used to detect biofilms on tissues and glass substrates showed up to 97 percent similarity, suggesting that the TB staining method is a promising technique for identifying biofilm regions.
Conclusions: The TB staining method demonstrates significant potential as an effective imaging technique for the identification of fluorescing and non-fluorescing biofilms on tissues and in wounds. This approach could lead to improved precision in surface-based treatments and better patient outcomes.
Keywords: biofilm imaging; image processing; segmentation; trypan blue; wounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

