BCL2 Inhibition Reveals a Dendritic Cell-Specific Immune Checkpoint That Controls Tumor Immunosurveillance
- PMID: 37623817
- PMCID: PMC7615270
- DOI: 10.1158/2159-8290.CD-22-1338
BCL2 Inhibition Reveals a Dendritic Cell-Specific Immune Checkpoint That Controls Tumor Immunosurveillance
Abstract
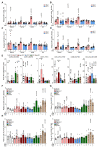
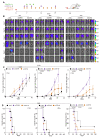
We developed a phenotypic screening platform for the functional exploration of dendritic cells (DC). Here, we report a genome-wide CRISPR screen that revealed BCL2 as an endogenous inhibitor of DC function. Knockout of BCL2 enhanced DC antigen presentation and activation as well as the capacity of DCs to control tumors and to synergize with PD-1 blockade. The pharmacologic BCL2 inhibitors venetoclax and navitoclax phenocopied these effects and caused a cDC1-dependent regression of orthotopic lung cancers and fibrosarcomas. Thus, solid tumors failed to respond to BCL2 inhibition in mice constitutively devoid of cDC1, and this was reversed by the infusion of DCs. Moreover, cDC1 depletion reduced the therapeutic efficacy of BCL2 inhibitors alone or in combination with PD-1 blockade and treatment with venetoclax caused cDC1 activation, both in mice and in patients. In conclusion, genetic and pharmacologic BCL2 inhibition unveils a DC-specific immune checkpoint that restrains tumor immunosurveillance.
Significance: BCL2 inhibition improves the capacity of DCs to stimulate anticancer immunity and restrain cancer growth in an immunocompetent context but not in mice lacking cDC1 or mature T cells. This study indicates that BCL2 blockade can be used to sensitize solid cancers to PD-1/PD-L1-targeting immunotherapy. This article is featured in Selected Articles from This Issue, p. 2293.
©2023 American Association for Cancer Research.
Conflict of interest statement
Figures





Comment in
-
Unleashing dendritic cell-mediated tumor clearance by targeting Bcl-2.Trends Immunol. 2023 Nov;44(11):871-873. doi: 10.1016/j.it.2023.09.007. Epub 2023 Oct 7. Trends Immunol. 2023. PMID: 37813733
-
Getting more bang for their buck: BCL2 inhibitors boost dendritic-cell function to enhance anti-cancer immune surveillance.J Transl Med. 2024 Mar 28;22(1):317. doi: 10.1186/s12967-024-04961-x. J Transl Med. 2024. PMID: 38549077 Free PMC article.
References
-
- Andre F, Filleron T, Kamal M, Mosele F, Arnedos M, Dalenc F, et al. Genomics to select treatment for patients with metastatic breast cancer. Nature. 2022;610(7931):343–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

