Metabolic Profile of Alzheimer's Disease: Is 10-Hydroxy-2-decenoic Acid a Pertinent Metabolic Adjuster?
- PMID: 37623897
- PMCID: PMC10456792
- DOI: 10.3390/metabo13080954
Metabolic Profile of Alzheimer's Disease: Is 10-Hydroxy-2-decenoic Acid a Pertinent Metabolic Adjuster?
Abstract
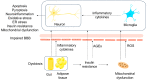
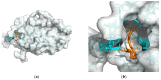
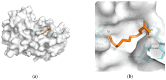
Alzheimer's disease (AD) represents a significant public health concern in modern society. Metabolic syndrome (MetS), which includes diabetes mellitus (DM) and obesity, represents a modifiable risk factor for AD. MetS and AD are interconnected through various mechanisms, such as mitochondrial dysfunction, oxidative stress, insulin resistance (IR), vascular impairment, inflammation, and endoplasmic reticulum (ER) stress. Therefore, it is necessary to seek a multi-targeted and safer approach to intervention. Thus, 10-hydroxy-2-decenoic acid (10-HDA), a unique hydroxy fatty acid in royal jelly, has shown promising anti-neuroinflammatory, blood-brain barrier (BBB)-preserving, and neurogenesis-promoting properties. In this paper, we provide a summary of the relationship between MetS and AD, together with an introduction to 10-HDA as a potential intervention nutrient. In addition, molecular docking is performed to explore the metabolic tuning properties of 10-HDA with associated macromolecules such as GLP-1R, PPARs, GSK-3, and TREM2. In conclusion, there is a close relationship between AD and MetS, and 10-HDA shows potential as a beneficial nutritional intervention for both AD and MetS.
Keywords: 10-HDA; Alzheimer’s disease; diabetes mellitus; molecular docking; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Nootropic effect of Indian Royal Jelly against okadaic acid induced rat model of Alzheimer's disease: Inhibition of neuroinflammation and acetylcholineesterase.J Tradit Complement Med. 2023 Nov 15;14(3):300-311. doi: 10.1016/j.jtcme.2023.11.005. eCollection 2024 May. J Tradit Complement Med. 2023. PMID: 38707922 Free PMC article.
-
Glucose metabolism enhancement by 10-hydroxy-2-decenoic acid via the PI3K/AKT signaling pathway in high-fat-diet/streptozotocin induced type 2 diabetic mice.Food Funct. 2022 Oct 3;13(19):9931-9946. doi: 10.1039/d1fo03818d. Food Funct. 2022. PMID: 36056641
-
Linking mitochondrial dysfunction, metabolic syndrome and stress signaling in Neurodegeneration.Biochim Biophys Acta Mol Basis Dis. 2017 May;1863(5):1132-1146. doi: 10.1016/j.bbadis.2016.06.015. Epub 2016 Jun 21. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27345267 Review.
-
Trans-10-hydroxy-2-decenoic acid alleviates LPS-induced blood-brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway.Eur J Pharmacol. 2019 Dec 15;865:172736. doi: 10.1016/j.ejphar.2019.172736. Epub 2019 Oct 12. Eur J Pharmacol. 2019. PMID: 31614141
-
Metabolic Syndrome and the Cellular Phase of Alzheimer's Disease.Prog Mol Biol Transl Sci. 2017;146:243-258. doi: 10.1016/bs.pmbts.2016.12.016. Epub 2017 Feb 4. Prog Mol Biol Transl Sci. 2017. PMID: 28253987 Review.
Cited by
-
Royal Jelly: Biological Action and Health Benefits.Int J Mol Sci. 2024 May 30;25(11):6023. doi: 10.3390/ijms25116023. Int J Mol Sci. 2024. PMID: 38892209 Free PMC article. Review.
-
Behind the Therapeutic Effects of Royal Jelly: Recent Advances in the Specific Properties of 10-Hydroxydecanoic Acid.Molecules. 2025 Jun 22;30(13):2694. doi: 10.3390/molecules30132694. Molecules. 2025. PMID: 40649213 Free PMC article. Review.
-
Impact of royal jelly consumption on oxidative stress, anti-oxidant markers and physical activities of patients with multiple sclerosis: a randomized double-blind placebo-controlled study.Ir J Med Sci. 2025 Aug 18. doi: 10.1007/s11845-025-04009-z. Online ahead of print. Ir J Med Sci. 2025. PMID: 40824557
-
Royal jelly acid: preparation, metabolism and therapeutic potential.Front Pharmacol. 2025 May 26;16:1561351. doi: 10.3389/fphar.2025.1561351. eCollection 2025. Front Pharmacol. 2025. PMID: 40492134 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

