Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams
- PMID: 37623908
- PMCID: PMC10456707
- DOI: 10.3390/metabo13080965
Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams
Abstract
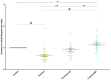
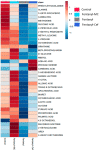
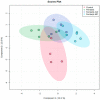
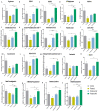
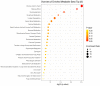
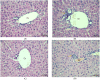
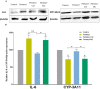
Fentanyl is a highly potent opioid analgesic that is approved medically to treat acute and chronic pain. There is a high potential for overdose-induced organ toxicities, including liver toxicity, and this might be due to the increase of recreational use of opioids. Several preclinical studies have demonstrated the efficacy of beta-lactams in modulating the expression of glutamate transporter-1 (GLT-1) in different body organs, including the liver. The upregulation of GLT-1 by beta-lactams is associated with the attenuation of hyperglutamatergic state, which is a characteristic feature of opioid use disorders. A novel experimental beta-lactam compound with no antimicrobial properties, MC-100093, has been developed to attenuate dysregulation of glutamate transport, in part by normalizing GLT-1 expression. A previous study showed that MC-100093 modulated hepatic GLT-1 expression with subsequent attenuation of alcohol-increased fat droplet content in the liver. In this study, we investigated the effects of fentanyl overdose on liver metabolites, and determined the effects of MC-100093 and ceftriaxone in the liver of a fentanyl overdose mouse model. Liver samples from control, fentanyl overdose, and fentanyl overdose ceftriaxone- or MC-100093-treated mice were analyzed for metabolomics using gas chromatography-mass spectrometry. Heatmap analysis revealed that both MC-100093 and ceftriaxone attenuated the effects of fentanyl overdose on several metabolites, and MC-100093 showed superior effects. Statistical analysis showed that MC-100093 reversed the effects of fentanyl overdose in some metabolites. Moreover, enrichment analysis revealed that the altered metabolites were strongly linked to the glucose-alanine cycle, the Warburg effect, gluconeogenesis, glutamate metabolism, lactose degradation, and ketone body metabolism. The changes in liver metabolites induced by fentanyl overdose were associated with liver inflammation, an effect attenuated with ceftriaxone pre-treatments. Ceftriaxone normalized fentanyl-overdose-induced changes in liver interleukin-6 and cytochrome CYP3A11 (mouse homolog of human CYP3A4) expression. Our data indicate that fentanyl overdose impaired liver metabolites, and MC-100093 restored certain metabolites.
Keywords: CYP-3A4; IL-6; MC-100093; beta-lactams; ceftriaxone; inflammation; metabolomic; opioids; overdose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
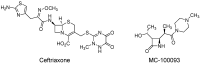
References
Grants and funding
LinkOut - more resources
Full Text Sources

