Extensive Drug Resistance of Strong Biofilm-Producing Acinetobacter baumannii Strains Isolated from Infections and Colonization Hospitalized Patients in Southern Poland
- PMID: 37623935
- PMCID: PMC10459043
- DOI: 10.3390/pathogens12080975
Extensive Drug Resistance of Strong Biofilm-Producing Acinetobacter baumannii Strains Isolated from Infections and Colonization Hospitalized Patients in Southern Poland
Abstract
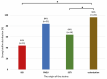
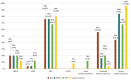
Acinetobacter baumannii (AB) is a bacterium that causes infections, particularly in immunocompromised patients. Treatment is challenging due to biofilm formation by AB strains, which hinders antibiotic effectiveness and promotes drug resistance. The aim of our study was to analyze the biofilm-producing capacity of AB isolates from various forms of infections in relation to biofilm-related genes and their drug resistance. We tested one hundred isolates for biofilm formation using the crystal violet microplate method. Drug resistance analyses were performed based on EUCAST and CLSI guidelines, and biofilm genes were detected using PCR. All tested strains were found to form biofilms, with 50% being ICU strains and 72% classified as strong biofilm producers. Among these, 87% were extensively drug-resistant (XDR) and 2% were extra-extensively drug-resistant (E-XDR). The most common gene set was bap, bfmS, csuE, and ompA, found in 57% of all isolates. Our research shows that, regardless of the form of infection, biofilm-forming strains can be expected among AB isolates. The emergence of E-XDR and XDR strains among non-ICU infections highlights the necessity for the rational use of antibiotics to stop or limit the further acquisition of drug resistance by A. baumannii.
Keywords: Acinetobacter baumannii; biofilm; drug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. [(accessed on 7 February 2023)]. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-....
-
- Høiby N., Bjarnsholt T., Moser C., Bassi G.L., Coenye T., Donelli G., Hall-Stoodley L., Holá V., Imbert C., Kirketerp-Møller K., et al. ESCMID Guideline for the Diagnosis and Treatment of Biofilm Infections 2014. Clin. Microbiol. Infect. 2015;21((Suppl. S1)):S1–S25. doi: 10.1016/j.cmi.2014.10.024. - DOI - PubMed
-
- Antimicrobial Resistance Surveillance in Europe 2022–2020. [(accessed on 7 February 2023)]. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance....
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

