Bioreactor Production Process of Spodoptera frugiperda multiple nucleopolyhedrovirus Biopesticide
- PMID: 37623961
- PMCID: PMC10457946
- DOI: 10.3390/pathogens12081001
Bioreactor Production Process of Spodoptera frugiperda multiple nucleopolyhedrovirus Biopesticide
Abstract
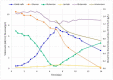
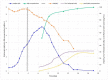
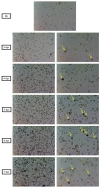
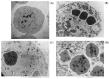
Spodoptera frugiperda (fall armyworm) is one of the most important maize pests in the world and the baculovirus Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV), a natural pathogen of this pest, has been used as a biopesticide for its control. At present, in vivo strategies at the commercial scale are employed by multiplying the virus in the host insect in biofactory facilities; however, in vitro large-scale production is an interesting alternative to overcome the limitations of baculoviruses massal production. This study aimed to develop the process of the SfMNPV in vitro production by evaluating the effects of different multiplicities of infection (MOI) and nutritional supplements, morphological and molecular analysis of the infection on the growth of Sf9 cells and virus production. The Bioreactor Stirred Tank Reactor (STR) approach with glutamine-supplemented Sf-900 III serum free culture medium, combined with the MOI of 1.0, showed the best viral production performance, with a specific productivity above 300 occlusion bodies (OBs)/cell and volumetric productivity of 9.0 × 1011 OBs/L.
Keywords: SfMNPV; Spodoptera frugiperda; baculovirus; biological control; bioreactor; fall armyworm; in vitro production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Farias J.R., Andow D.A., Horikoshi R.J., Sorgatto R.J., Fresia P., Dos Santos A.C., Omoto C. Field evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014;64:150–158. doi: 10.1016/j.cropro.2014.06.019. - DOI
-
- Cruz I., Figueiredo M., Oliveira A.C., Vasconcelos C.A. Damage of Spodoptera frugiperda (Smith) in different maize genotypes cultivated in soil under three levels of aluminum saturation. Int. J. Pest. Manag. 1999;45:293–296. doi: 10.1080/096708799227707. - DOI
-
- Goergen G., Kumar P.L., Sankung S.B., Togola A., Tamo M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and Central Africa. PLoS ONE. 2016;11:e0165632. doi: 10.1371/journal.pone.0165632. - DOI - PMC - PubMed
-
- Wang R., Jiang C., Guo X., Chen D., You C., Zhang Y., Wang M., Li Q. Potential distribution of Spodoptera frugiperda (J.E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Conserv. 2020;21:e00865. doi: 10.1016/j.gecco.2019.e00865. - DOI
-
- Miranda G.R.B., Raetano C.G., Silva E., Daam M.A., Cerejeira M.J. Environmental fate of neonicotinoids and classification of their potential risks to hypogean, epygean, and surface water ecosystems in Brazil. Hum. Ecol. Risk Assess. 2011;17:981–995. doi: 10.1080/10807039.2011.588159. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

