Therapeutic Potential of Engineered Virus-like Particles of Parvovirus B19
- PMID: 37623967
- PMCID: PMC10458557
- DOI: 10.3390/pathogens12081007
Therapeutic Potential of Engineered Virus-like Particles of Parvovirus B19
Abstract
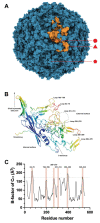
Virus-like particles (VLPs) comprise one or many structural components of virions, except their genetic material. Thus, VLPs keep their structural properties of cellular recognition while being non-infectious. VLPs of Parvovirus B19 (B19V) can be produced by the heterologous expression of their structural proteins VP1 and VP2 in bacteria. These proteins are purified under denaturing conditions, refolded, and assembled into VLPs. Moreover, chimeric forms of VP2 have been constructed to harbor peptides or functional proteins on the surface of the particles without dropping their competence to form VLPs, serving as presenting nanoparticles. The in-vitro assembly approach offers exciting possibilities for the composition of VLPs, as more than one chimeric form of VP2 can be included in the assembly stage, producing multifunctional VLPs. Here, the heterologous expression and in-vitro assembly of B19V structural proteins and their chimeras are reviewed. Considerations for the engineering of the structural proteins of B19V are also discussed. Finally, the construction of multifunctional VLPs and their future potential as innovative medical tools are examined.
Keywords: enzyme nanocarriers; nanobiotecnology; nanomedicine; parvovirus-like particles; protein engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures
References
-
- Johnston G.P., Bradel-Tretheway B., Piehowski P.D., Brewer H.M., Lee B.N.R., Usher N.T., Zamora J.L.R., Ortega V., Contreras E.M., Teuton J.R., et al. Nipah Virus-Like Particle Egress Is Modulated by Cytoskeletal and Vesicular Trafficking Pathways: A Validated Particle Proteomics Analysis. mSystems. 2019;4:e00194-19. doi: 10.1128/mSystems.00194-19. - DOI - PMC - PubMed
-
- Saiding Q., Zhao D., Chen X., Cui W. Antigens-on-a-Ship: A New Journey to Solid Tumor Vaccines. Matter. 2023;6:1680–1682. doi: 10.1016/j.matt.2023.04.011. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

