Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model
- PMID: 37624009
- PMCID: PMC10458499
- DOI: 10.3390/pathogens12081049
Taurine, a Component of the Tear Film, Exacerbates the Pathogenic Mechanisms of Acanthamoeba castellanii in the Ex Vivo Amoebic Keratitis Model
Abstract
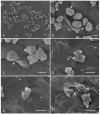
Acanthamoeba spp. is the etiological agent of amoebic keratitis. In this study, the effect of taurine in physiological concentrations in tears (195 μM) on trophozoites of Acanthamoeba castellanii through the ex vivo amoebic keratitis model was evaluated. Trophozoites were coincubated with the Syrian golden hamster cornea (Mesocricetus auratus) for 3 and 6 h. Group 1: Control (-). Corneas coincubated with amoebic culture medium and taurine. Group 2: Control (+). Corneas coincubated with trophozoites without taurine. Group 3: Corneas coincubated with taurine 15 min before adding trophozoites. Group 4: Trophozoites coincubated 15 min with taurine before placing them on the cornea. Group 5: Corneas coincubated for 15 min with trophozoites; subsequently, taurine was added. Results are similar for both times, as evaluated by scanning electron microscopy. As expected, in the corneas of Group 1, no alterations were observed in the corneal epithelium. In the corneas of Group 2, few adhered trophozoites were observed on the corneal surface initiating migrations through cell junctions as previously described; however, in corneas of Groups 3, 4 and 5, abundant trophozoites were observed, penetrating through different corneal cell areas, emitting food cups and destabilizing corneal surface in areas far from cell junctions. Significant differences were confirmed in trophozoites adherence coincubated with taurine (p < 0.05). Taurine does not prevent the adhesion and invasion of the amoebae, nor does it favor its detachment once these have adhered to the cornea, suggesting that taurine in the physiological concentrations found in tears stimulates pathogenic mechanisms of A. castellanii.
Keywords: A. castellanii; pathogenic mechanisms; taurine in tear.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Staurosporine as a Potential Treatment for Acanthamoeba Keratitis Using Mouse Cornea as an Ex Vivo Model.Mar Drugs. 2024 Sep 18;22(9):423. doi: 10.3390/md22090423. Mar Drugs. 2024. PMID: 39330304 Free PMC article.
-
Acanthamoeba castellanii: Effect of neuroactive substances on trophozoite migration.Exp Parasitol. 2022 May-Jun;236-237:108245. doi: 10.1016/j.exppara.2022.108245. Epub 2022 Mar 10. Exp Parasitol. 2022. PMID: 35283169
-
Acanthamoeba castellanii trophozoites that survive multipurpose solutions are able to adhere to cosmetic contact lenses, increasing the risk of infection.Heliyon. 2023 Aug 29;9(9):e19599. doi: 10.1016/j.heliyon.2023.e19599. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809484 Free PMC article.
-
Silicone hydrogel contact lenses surface promote Acanthamoeba castellanii trophozoites adherence: qualitative and quantitative analysis.Eye Contact Lens. 2014 May;40(3):132-9. doi: 10.1097/ICL.0000000000000024. Eye Contact Lens. 2014. PMID: 24699779
-
Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea.PLoS One. 2014 Mar 14;9(3):e92375. doi: 10.1371/journal.pone.0092375. eCollection 2014. PLoS One. 2014. PMID: 24633052 Free PMC article.
Cited by
-
Staurosporine as a Potential Treatment for Acanthamoeba Keratitis Using Mouse Cornea as an Ex Vivo Model.Mar Drugs. 2024 Sep 18;22(9):423. doi: 10.3390/md22090423. Mar Drugs. 2024. PMID: 39330304 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

