The Therapeutic Potential of Angeli's Salt in Mitigating Acute Trypanosoma cruzi Infection in Mice
- PMID: 37624023
- PMCID: PMC10458646
- DOI: 10.3390/pathogens12081063
The Therapeutic Potential of Angeli's Salt in Mitigating Acute Trypanosoma cruzi Infection in Mice
Abstract
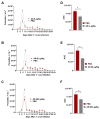
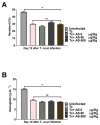
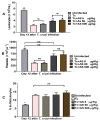
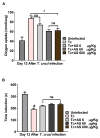
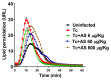
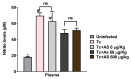
Chagas disease (CD), caused by Trypanosoma cruzi, is a neglected tropical disease prevalent in Latin America. Infected patients are treated to eliminate the parasite, reduce the cardiomyopathy risk, and interrupt the disease transmission cycle. The World Health Organization recognizes benznidazole (BZ) and nifurtimox as effective drugs for CD treatment. In the chronic phase, both drugs have low cure rates and serious side effects. T. cruzi infection causes intense tissue inflammation that controls parasite proliferation and CD evolution. Compounds that liberate nitric oxide (NO) (NO donors) have been used as anti-T. cruzi therapeutics. Currently, there is no evidence that nitroxyl (HNO) affects T. cruzi infection outcomes. This study investigated the effects of the HNO donor Angeli's salt (AS) on C57BL/6 mice infected with T. cruzi (Y strain, 5 × 103 trypomastigotes, intraperitoneally). AS reduced the number of parasites in the bloodstream and heart nests and increased the protective antioxidant capacity of erythrocytes in infected animals, reducing disease severity. Furthermore, in vitro experiments showed that AS treatment reduced parasite uptake and trypomastigote release by macrophages. Taken together, these findings from the murine model and in vitro testing suggest that AS could be a promising therapy for CD.
Keywords: Chagas disease; leukopenia; macrophages; nitroxyl; oxidative stress; therapy; thrombocytopenia.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



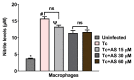
References
-
- WHO Chagas Disease (Also Known as American Trypanosomiasis) 2023. [(accessed on 15 June 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america...
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

