Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems
- PMID: 37624095
- PMCID: PMC10908410
- DOI: 10.1152/ajpheart.00288.2023
Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems
Abstract
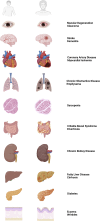
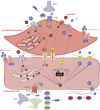
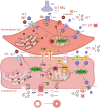
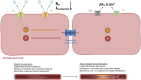
Individuals aged ≥65 yr will comprise ∼20% of the global population by 2030. Cardiovascular disease remains the leading cause of death in the world with age-related endothelial "dysfunction" as a key risk factor. As an organ in and of itself, vascular endothelium courses throughout the mammalian body to coordinate blood flow to all other organs and tissues (e.g., brain, heart, lung, skeletal muscle, gut, kidney, skin) in accord with metabolic demand. In turn, emerging evidence demonstrates that vascular aging and its comorbidities (e.g., neurodegeneration, diabetes, hypertension, kidney disease, heart failure, and cancer) are "channelopathies" in large part. With an emphasis on distinct functional traits and common arrangements across major organs systems, the present literature review encompasses regulation of vascular ion channels that underlie blood flow control throughout the body. The regulation of myoendothelial coupling and local versus conducted signaling are discussed with new perspectives for aging and the development of chronic diseases. Although equipped with an awareness of knowledge gaps in the vascular aging field, a section has been included to encompass general feasibility, role of biological sex, and additional conceptual and experimental considerations (e.g., cell regression and proliferation, gene profile analyses). The ultimate goal is for the reader to see and understand major points of deterioration in vascular function while gaining the ability to think of potential mechanistic and therapeutic strategies to sustain organ perfusion and whole body health with aging.
Keywords: K+ channels; TRP channels; endothelial function; myoendothelial coupling; vascular aging.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

