Arsenic Impairs Differentiation of Human Induced Pluripotent Stem Cells into Cholinergic Motor Neurons
- PMID: 37624150
- PMCID: PMC10458826
- DOI: 10.3390/toxics11080644
Arsenic Impairs Differentiation of Human Induced Pluripotent Stem Cells into Cholinergic Motor Neurons
Abstract
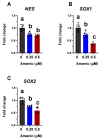
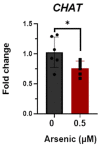
Arsenic exposure during embryogenesis can lead to improper neurodevelopment and changes in locomotor activity. Additionally, in vitro studies have shown that arsenic inhibits the differentiation of sensory neurons and skeletal muscle. In the current study, human-induced pluripotent stem (iPS) cells were differentiated into motor neurons over 28 days, while being exposed to up to 0.5 μM arsenic. On day 6, neuroepithelial progenitor cells (NEPs) exposed to arsenic had reduced transcript levels of the neural progenitor/stem cell marker nestin (NES) and neuroepithelial progenitor marker SOX1, while levels of these transcripts were increased in motor neuron progenitors (MNPs) at day 12. In day 18 early motor neurons (MNs), choline acetyltransferase (CHAT) expression was reduced two-fold in cells exposed to 0.5 μM arsenic. RNA sequencing demonstrated that the cholinergic synapse pathway was impaired following exposure to 0.5 μM arsenic, and that transcript levels of genes involved in acetylcholine synthesis (CHAT), transport (solute carriers, SLC18A3 and SLC5A7) and degradation (acetylcholinesterase, ACHE) were all downregulated in day 18 early MNs. In day 28 mature motor neurons, arsenic significantly downregulated protein expression of microtubule-associated protein 2 (MAP2) and ChAT by 2.8- and 2.1-fold, respectively, concomitantly with a reduction in neurite length. These results show that exposure to environmentally relevant arsenic concentrations dysregulates the differentiation of human iPS cells into motor neurons and impairs the cholinergic synapse pathway, suggesting that exposure impairs cholinergic function in motor neurons.
Keywords: ChAT; arsenic; cholinergic synapse; human induced pluripotent stem cells; motor neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Olig2 positive cells derived from hair follicle neural crest stem cells in rats.J Chem Neuroanat. 2020 Apr;105:101770. doi: 10.1016/j.jchemneu.2020.101770. Epub 2020 Feb 20. J Chem Neuroanat. 2020. PMID: 32088378
-
Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells.Mol Brain. 2015 Dec 1;8(1):79. doi: 10.1186/s13041-015-0172-4. Mol Brain. 2015. PMID: 26626025 Free PMC article.
-
Global transcriptome profile of the developmental principles of in vitro iPSC-to-motor neuron differentiation.BMC Mol Cell Biol. 2021 Feb 18;22(1):13. doi: 10.1186/s12860-021-00343-z. BMC Mol Cell Biol. 2021. PMID: 33602141 Free PMC article.
-
Directed differentiation of basal forebrain cholinergic neurons from human pluripotent stem cells.J Neurosci Methods. 2016 Jun 15;266:42-9. doi: 10.1016/j.jneumeth.2016.03.017. Epub 2016 Mar 29. J Neurosci Methods. 2016. PMID: 27036311
-
Molecular forms of acetylcholinesterases in Alzheimer's disease.Fed Proc. 1986 Dec;45(13):2982-8. Fed Proc. 1986. PMID: 2430839 Review.
References
-
- Mochizuki H., Phyu K.P., Aung M.N., Zin P.W., Yano Y., Myint M.Z., Thit W.M., Yamamoto Y., Hishikawa Y., Thant K.Z., et al. Peripheral neuropathy induced by drinking water contaminated with low-dose arsenic in Myanmar. Environ. Health Prev. Med. 2019;24:23. doi: 10.1186/s12199-019-0781-0. - DOI - PMC - PubMed
-
- Bhattacharya P., Hossain M., Rahman S.N., Robinson C., Nath B., Rahman M., Islam M.M., Von Bromssen M., Ahmed K.M., Jacks G., et al. Temporal and seasonal variability of arsenic in drinking water wells in Matlab, southeastern Bangladesh: A preliminary evaluation on the basis of a 4 year study. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2011;46:1177–1184. doi: 10.1080/10934529.2011.598768. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

