In Silico-Ex Vitro Iteration Strategy for Affinity Maturation of Anti-Ricin Peptides and the SPR Biosensing Application
- PMID: 37624247
- PMCID: PMC10467137
- DOI: 10.3390/toxins15080490
In Silico-Ex Vitro Iteration Strategy for Affinity Maturation of Anti-Ricin Peptides and the SPR Biosensing Application
Abstract
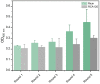
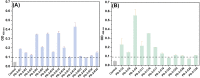
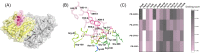
The highly toxic plant toxin ricin is one of the most known threatening toxins. Accurate and sensitive biosensing methods for the first emergency response and intoxication treatment, are always pursued in the biodefense field. Screening affinity molecules is the fundamental mainstream approach for developing biosensing methods. Compared with common affinity molecules such as antibodies and oligonucleotide aptamers, peptides have great potential as biosensing modules with more accessible chemical synthesis capability and better batch-to-batch stability than antibodies, more abundant interaction sites, and robust sensing performance towards complex environments. However, anti-ricin peptides are so scant to be screened and discovered, and an advanced screening strategy is the utmost to tackle this issue. Here, we present a new in silico-in vitro iteration-assisted affinity maturation strategy of anti-ricin peptides. We first obtained affinity peptides targeting ricin through phage display with five panning rounds of "coating-elution-amplification-enrichment" procedures. The binding affinity and kinetic parameters characterized by surface plasmon resonance (SPR) showed that we had obtained four peptides owning dissociation constants (KD) around 2~35 μM, in which peptide PD-2-R5 has the lower KD of 4.7 μM and higher stable posture to interact with ricin. We then constructed a new strategy for affinity maturity, composing two rounds of in silico-in vitro iterations. Firstly, towards the single-site alanine scanning mutation peptide library, the molecular docking predictions match the SPR evaluation results well, laying a solid foundation for designing a full saturation mutated peptide library. Secondly, plenty of in silico saturation mutation prediction results guided the discovery of peptides PD2-R5-T3 and PD-2-R5-T4 with higher affinity from only a limited number of SPR evaluation experiments. Both evolved peptides had increased affinity by about 5~20 times, i.e., KD of 230 nM and 900 nM. A primary cellular toxicity assay indicated that both peptides could protect cells against ricin damage. We further established an SPR assay based on PD-2-R5-T3 and PD-2-R5-T4 elongated with an antifouling peptide linkage and achieved good linearity with a sensitivity of 1 nM and 0.5 nM, respectively. We hope this new affinity-mature strategy will find its favorable position in relevant peptide evolution, biosensing, and medical countermeasures for biotoxins to protect society's security and human life better.
Keywords: affinity maturation; biosensing; molecular docking; peptide; phage display; ricin; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
In silico panning for a non-competitive peptide inhibitor.BMC Bioinformatics. 2007 Jan 12;8:11. doi: 10.1186/1471-2105-8-11. BMC Bioinformatics. 2007. PMID: 17222344 Free PMC article.
-
Label-free differentiation and quantification of ricin, abrin from their agglutinin biotoxins by surface plasmon resonance.Talanta. 2022 Feb 1;238(Pt 1):122860. doi: 10.1016/j.talanta.2021.122860. Epub 2021 Sep 9. Talanta. 2022. PMID: 34857316
-
Improved antibody-based ricin neutralization by affinity maturation is correlated with slower off-rate values.Protein Eng Des Sel. 2017 Sep 1;30(9):611-617. doi: 10.1093/protein/gzx028. Protein Eng Des Sel. 2017. PMID: 28472478
-
Recent Advancements in Aptamer-Based Surface Plasmon Resonance Biosensing Strategies.Biosensors (Basel). 2021 Jul 10;11(7):233. doi: 10.3390/bios11070233. Biosensors (Basel). 2021. PMID: 34356703 Free PMC article. Review.
-
Affinity-Based Nanoarchitectured Biotransducer for Sensitivity Enhancement of Surface Plasmon Resonance Sensors for In Vitro Diagnosis: A Review.ACS Biomater Sci Eng. 2021 Jan 11;7(1):2-30. doi: 10.1021/acsbiomaterials.0c01203. Epub 2020 Dec 5. ACS Biomater Sci Eng. 2021. PMID: 33455205 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

