Unveiling the Protein Components of the Secretory-Venom Gland and Venom of the Scorpion Centruroides possanii (Buthidae) through Omic Technologies
- PMID: 37624255
- PMCID: PMC10467079
- DOI: 10.3390/toxins15080498
Unveiling the Protein Components of the Secretory-Venom Gland and Venom of the Scorpion Centruroides possanii (Buthidae) through Omic Technologies
Abstract
Centruroides possanii is a recently discovered species of "striped scorpion" found in Mexico. Certain species of Centruroides are known to be toxic to mammals, leading to numerous cases of human intoxications in the country. Venom components are thought to possess therapeutic potential and/or biotechnological applications. Hence, obtaining and analyzing the secretory gland transcriptome and venom proteome of C. possanii is relevant, and that is what is described in this communication. Since this is a newly described species, first, its LD50 to mice was determined and estimated to be 659 ng/g mouse weight. Using RNA extracted from this species and preparing their corresponding cDNA fragments, a transcriptome analysis was obtained on a Genome Analyzer (Illumina) using the 76-base pair-end sequencing protocol. Via high-throughput sequencing, 19,158,736 reads were obtained and ensembled in 835,204 sequences. Of them, 28,399 transcripts were annotated with Pfam. A total of 244 complete transcripts were identified in the transcriptome of C. possanii. Of these, 109 sequences showed identity to toxins that act on ion channels, 47 enzymes, 17 protease inhibitors (PINs), 11 defense peptides (HDPs), and 60 in other components. In addition, a sample of the soluble venom obtained from this scorpion was analyzed using an Orbitrap Velos apparatus, which allowed for identification by liquid chromatography followed by mass spectrometry (LC-MS/MS) of 70 peptides and proteins: 23 toxins, 27 enzymes, 6 PINs, 3 HDPs, and 11 other components. Until now, this work has the highest number of scorpion venom components identified through omics technologies. The main novel findings described here were analyzed in comparison with the known data from the literature, and this process permitted some new insights in this field.
Keywords: Centruroides possanii; next generation sequencing; omics technologies; proteome; scorpion; transcriptome; venom; venom gland.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


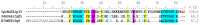



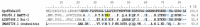

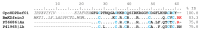
Similar articles
-
Dissecting Toxicity: The Venom Gland Transcriptome and the Venom Proteome of the Highly Venomous Scorpion Centruroides limpidus (Karsch, 1879).Toxins (Basel). 2019 Apr 30;11(5):247. doi: 10.3390/toxins11050247. Toxins (Basel). 2019. PMID: 31052267 Free PMC article.
-
Venom-gland transcriptomics and venom proteomics of the Hentz striped scorpion (Centruroides hentzi; Buthidae) reveal high toxin diversity in a harmless member of a lethal family.Toxicon. 2018 Feb;142:14-29. doi: 10.1016/j.toxicon.2017.12.042. Epub 2017 Dec 14. Toxicon. 2018. PMID: 29248469
-
Transcriptomic and proteomic analyses of the venom and venom glands of Centruroides hirsutipalpus, a dangerous scorpion from Mexico.Toxicon. 2020 May;179:21-32. doi: 10.1016/j.toxicon.2020.02.021. Epub 2020 Feb 29. Toxicon. 2020. PMID: 32126222
-
Scorpion venomics: a 2019 overview.Expert Rev Proteomics. 2020 Jan;17(1):67-83. doi: 10.1080/14789450.2020.1705158. Epub 2019 Dec 25. Expert Rev Proteomics. 2020. PMID: 31834817 Review.
-
Mining on scorpion venom biodiversity.Toxicon. 2010 Dec 15;56(7):1155-61. doi: 10.1016/j.toxicon.2009.11.010. Epub 2009 Nov 18. Toxicon. 2010. PMID: 19931296 Review.
Cited by
-
Characterization of Sodium Channel Peptides Obtained from the Venom of the Scorpion Centruroides bonito.Toxins (Basel). 2024 Mar 1;16(3):125. doi: 10.3390/toxins16030125. Toxins (Basel). 2024. PMID: 38535792 Free PMC article.
-
Transcriptomics-Informed Proteomics of Venom Glands and Crude Venom from Tityus cf. asthenes from Panama: Enzymes, Proteins, Toxins, and Antimicrobial Peptides.J Proteome Res. 2025 Jun 6;24(6):2906-2915. doi: 10.1021/acs.jproteome.5c00125. Epub 2025 May 28. J Proteome Res. 2025. PMID: 40435298
References
-
- Lourenço W.R. Scorpion Venoms. Springer; Dordrecht, The Netherlands: 2015. Scorpion Diversity and Distribution: Past and Present Patterns; pp. 3–23.
-
- Rein J. Norwegian University of Science and Technology; Trondheim, Norway: 2022. [(accessed on 29 May 2023)]. The Scorpion Files. Available online: https://www.ntnu.no/ub/scorpion-files/
-
- Shao J., Zhang R., Ge X., Yang B., Zhang J. Analgesic Peptides in Buthus martensii Karsch: A Traditional Chinese Animal Medicine. Asian J. Tradit. Med. 2007;2:45–50.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

