A Multi-Layer-Controlled Strategy for Cloning and Expression of Toxin Genes in Escherichia coli
- PMID: 37624265
- PMCID: PMC10467106
- DOI: 10.3390/toxins15080508
A Multi-Layer-Controlled Strategy for Cloning and Expression of Toxin Genes in Escherichia coli
Abstract
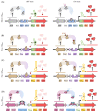
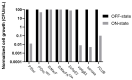
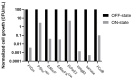
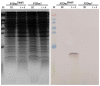
Molecular cloning and controlled expression remain challenging when the target gene encodes a protein that is toxic to the host. We developed a set of multi-layer control systems to enable cloning of genes encoding proteins known to be highly toxic in Escherichia coli and other bacteria. The different multi-layer control systems combine a promoter-operator system on a transcriptional level with a riboswitch for translational control. Additionally, replicational control is ensured by using a strain that reduces the plasmid copy number. The use of weaker promoters (such as PBAD or PfdeA) in combination with the effective theophylline riboswitch is essential for cloning genes that encode notoriously toxic proteins that directly target translation and transcription. Controlled overexpression is possible, allowing the system to be used for evaluating in vivo effects of the toxin. Systems with a stronger promoter can be used for successful overexpression and purification of the desired protein but are limited to toxins that are more moderate and do not interfere with their own production.
Keywords: cloning; replicational control; riboswitch; toxins; transcriptional control; translational control.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Translational gene expression control in Chlamydia trachomatis.PLoS One. 2022 Jan 27;17(1):e0257259. doi: 10.1371/journal.pone.0257259. eCollection 2022. PLoS One. 2022. PMID: 35085261 Free PMC article.
-
Fluoride-Controlled Riboswitch-Based Dampening of Gene Expression for Cloning Potent Promoters.Front Genet. 2022 Jan 21;12:591543. doi: 10.3389/fgene.2021.591543. eCollection 2021. Front Genet. 2022. PMID: 35126444 Free PMC article.
-
Tightly regulated, high-level expression from controlled copy number vectors based on the replicon of temperate phage N15.Gene. 2007 Jun 15;395(1-2):15-21. doi: 10.1016/j.gene.2006.12.036. Epub 2007 Jan 20. Gene. 2007. PMID: 17433573
-
Extremely Low Leakage Expression Systems Using Dual Transcriptional-Translational Control for Toxic Protein Production.Int J Mol Sci. 2020 Jan 21;21(3):705. doi: 10.3390/ijms21030705. Int J Mol Sci. 2020. PMID: 31973139 Free PMC article. Review.
-
Back to basics: pBR322 and protein expression systems in E. coli.Methods Mol Biol. 2004;267:77-90. doi: 10.1385/1-59259-774-2:077. Methods Mol Biol. 2004. PMID: 15269416 Review.
Cited by
-
De novo gene synthesis by an antiviral reverse transcriptase.bioRxiv [Preprint]. 2024 May 8:2024.05.08.593200. doi: 10.1101/2024.05.08.593200. bioRxiv. 2024. Update in: Science. 2024 Oct 4;386(6717):eadq0876. doi: 10.1126/science.adq0876. PMID: 38766058 Free PMC article. Updated. Preprint.
-
A CRISPR-Cas9 System for Knock-out and Knock-in of High Molecular Weight DNA Enables Module-Swapping of the Pikromycin Synthase in its Native Host.Res Sq [Preprint]. 2025 Mar 27:rs.3.rs-6229288. doi: 10.21203/rs.3.rs-6229288/v1. Res Sq. 2025. Update in: Microb Cell Fact. 2025 May 27;24(1):125. doi: 10.1186/s12934-025-02741-w. PMID: 40195982 Free PMC article. Updated. Preprint.
-
De novo gene synthesis by an antiviral reverse transcriptase.Science. 2024 Oct 4;386(6717):eadq0876. doi: 10.1126/science.adq0876. Epub 2024 Oct 4. Science. 2024. PMID: 39116258 Free PMC article.
-
A CRISPR-Cas9 system for knock-out and knock-in of high molecular weight DNA enables module-swapping of the pikromycin synthase in its native host.Microb Cell Fact. 2025 May 27;24(1):125. doi: 10.1186/s12934-025-02741-w. Microb Cell Fact. 2025. PMID: 40426207 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

