Understanding the Immunomodulatory Effects of Bovine Colostrum: Insights into IL-6/IL-10 Axis-Mediated Inflammatory Control
- PMID: 37624306
- PMCID: PMC10458264
- DOI: 10.3390/vetsci10080519
Understanding the Immunomodulatory Effects of Bovine Colostrum: Insights into IL-6/IL-10 Axis-Mediated Inflammatory Control
Abstract
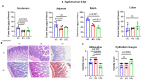
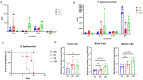
Bovine colostrum (COL), the first milk secreted by lactating cows postpartum, is a rich source of bioactive compounds that exert a significant role in the survival, growth, and immune development of neonatal calves. This study investigated the immunomodulatory effects of COL on cytokine production in vitro using a Caco-2/THP-1 macrophage co-culture model stimulated with Phorbol 12-myristate 13-acetate (PMA). COL pretreatment significantly reduced IL-6 (241.3 pg/mL) production induced by PMA (p < 0.05), while increasing IL-10 production (45.3 pg/mL), in comparison to PMA control (441.1 and 12.5 pg/mL, respectively). Further investigations revealed that the IL-6 suppressive effect of colostrum was heat-sensitive and associated with components of higher molecular mass (100 kDa). Moreover, colostrum primarily influenced THP-1 macrophages rather than Caco-2 epithelial cells. The effects of colostrum on IL-6 production were associated with reduced NF-κB activation in THP-1 macrophages. In calf-FMT transplanted C57BL/6 murine model, colostrum decreased intestinal permeability, reduced immune cell infiltration and intestinal score, and suppressed IL-6 (142.0 pg/mL) production during S. typhimurium infection, in comparison to control animals (215.2 pg/mL). These results suggest the immunomodulatory activity of bovine colostrum and its potential applications in inflammatory disorders. Further studies are needed to elucidate the underlying mechanisms and validate the findings in bovine models.
Keywords: Caco-2 cells; NF-κB; S. typhimurium infection; THP-1 macrophages; bovine colostrum; cytokine production; immunomodulation; inflammatory disorders; intestinal permeability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Poonia A. Shiva Bioactive Compounds, Nutritional Profile and Health Benefits of Colostrum: A Review. Food Prod. Process. Nutr. 2022;4:26. doi: 10.1186/s43014-022-00104-1. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

