Transcatheter interventions for left-sided valvular heart disease complicated by cardiogenic shock: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the Association for Acute Cardiovascular Care (ACVC) and the ESC Working Group on Cardiovascular Surgery
- PMID: 37624587
- PMCID: PMC10587846
- DOI: 10.4244/EIJ-D-23-00473
Transcatheter interventions for left-sided valvular heart disease complicated by cardiogenic shock: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the Association for Acute Cardiovascular Care (ACVC) and the ESC Working Group on Cardiovascular Surgery
Abstract
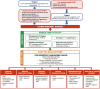
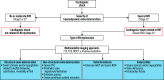
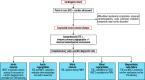
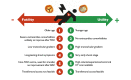
Valvular heart disease (VHD) is one of the most frequent causes of heart failure (HF) and is associated with poor prognosis, particularly among patients with conservative management. The development and improvement of catheter-based VHD interventions have broadened the indications for transcatheter valve interventions from inoperable/high-risk patients to younger/lower-risk patients. Cardiogenic shock (CS) associated with severe VHD is a clinical condition with a very high risk of mortality for which surgical treatment is often deemed a prohibitive risk. Transcatheter valve interventions might be a promising alternative in this setting given that they are less invasive. However, supportive scientific evidence is scarce and often limited to small case series. Current guidelines on VHD do not contain specific recommendations on how to manage patients with both VHD and CS. The purpose of this clinical consensus statement, developed by a group of international experts invited by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee, is to perform a review of the available scientific evidence on the management of CS associated with left-sided VHD and to provide a rationale and practical approach for the application of transcatheter valve interventions in this specific clinical setting.
Conflict of interest statement
N. Bonaros reports grants from Edwards Lifesciences and Corcym; and lecture fees from Edwards Lifesciences and Medtronic. P. Carrilho-Ferreira reports lecture fees from AstraZeneca, A. Menarini Diagnostics, Bayer, Biotronik, Medinfar, and Medtronic; and serves on an advisory board for Medtronic. M. Czerny is a consultant for Terumo Aortic, Medtronic, Endospan, and NEOS; and is a shareholder of TEVAR Ltd and Ascense Medical. C. Fraccaro reports support for attending meetings from Medtronic. C. Hassager reports research grants from the Novo Nordisk Foundation and the Lundbeck Foundation; and lecture honorarium from Abiomed. N. Karam reports consulting and lecture fees from Medtronic, Edwards Lifesciences, and Abbott Vascular. W-K. Kim reports lecture fees and honoraria from Abbott, Boston Scientific, Meril Life Sciences, Edwards Lifesciences, Medtronic, and Shockwave Medical. K.A. Krychtiuk reports lecture and/or consulting fees from Amgen, Novartis, and Sanofi. H. Möllmann received speaker honoraria/proctor fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. J. Pręgowski reports lecture fees from Abbott and Edwards Lifesciences; and contracts from Abbott. G. Tarantini reports lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, Boston Scientific, GADA, and Abiomed. J. Ternacle reports consulting fees from Abbott, GE HealthCare, and Philips; and lecture fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Figures
References
-
- Tabata N, Sinning JM, Kaikita K, Tsujita K, Nickenig G, Werner N. Current status and future perspective of structural heart disease intervention. J Cardiol. 2019;74:1–12. - PubMed
-
- Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. - PubMed
-
- Bohula EA, Katz JN, Diepen S, Alviar CL, Baird-Zars VM, Park JG, Barnett CF, Bhattal G, Barsness GW, Burke JA, Cremer PC, Cruz J, Daniels LB, DeFilippis A, Granger CB, Hollenberg S, Horowitz JM, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Ng J, Orgel R, Overgaard CB, Phreaner N, Roswell RO, Schulman SP, Snell RJ, Solomon MA, Ternus B, Tymchak W, Vikram F, Morrow DA Critical Care Cardiology Trials Network. Demographics, Care Patterns, and Outcomes of Patients Admitted to Cardiac Intensive Care Units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019;4:928–35. - PMC - PubMed
-
- Tavazzi G, Rossello X, Grand J, Gierlotka M, Sionis A, Ahrens I, Hassager C, Price S. Epidemiology, monitoring, and treatment strategy in cardiogenic shock. A multinational cross-sectional survey of ESC-acute cardiovascular care association research section. Eur Heart J Acute Cardiovasc Care. 2022;11:706–11. - PubMed
-
- Bhatt AS, Berg DD, Bohula EA, Alviar CL, Baird-Zars VM, Barnett CF, Burke JA, Carnicelli AP, Chaudhry SP, Daniels LB, Fang JC, Fordyce CB, Gerber DA, Guo J, Jentzer JC, Katz JN, Keller N, Kontos MC, Lawler PR, Menon V, Metkus TS, Nativi-Nicolau J, Phreaner N, Roswell RO, Sinha SS, Jeffrey Snell, Solomon MA, Van Diepen, Morrow DA. De Novo vs Acute-on-Chronic Presentations of Heart Failure-Related Cardiogenic Shock: Insights from the Critical Care Cardiology Trials Network Registry. J Card Fail. 2021;27:1073–81. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

