Efficacy and Safety of the Smallpox Vaccine for Postexposure Prophylaxis in Monkeypox: Protocol for an Open-Labeled, Single-Armed Study
- PMID: 37624623
- PMCID: PMC10492167
- DOI: 10.2196/46955
Efficacy and Safety of the Smallpox Vaccine for Postexposure Prophylaxis in Monkeypox: Protocol for an Open-Labeled, Single-Armed Study
Abstract
Background: In May 2022, a case of monkeypox (currently known as "mpox") with no history of overseas travel was reported in the United Kingdom, followed by reports of infections reported in Europe, the United States, and other countries worldwide. Due to the significant overlap in immune responses among viruses of the genus Orthopoxvirus (including smallpox virus, mpox virus, and vaccinia virus), it is believed that cross-immunity can be achieved by administering the smallpox virus vaccine. In Japan, a smallpox vaccine (LC16m8 strain vaccine) has been approved; however, there was no regulatory approval for the mpox vaccine during the design of this study. Although it is believed that individuals exposed to the mpox virus may receive smallpox vaccination as mpox prophylaxis, the existing evidence is not clear.
Objective: The primary objective was to evaluate the efficacy of the LC16m8 strain vaccine, approved for smallpox in Japan, for postexposure prophylaxis against mpox when administered to close contacts of individuals with mpox. The secondary objective was to investigate the safety of the vaccine for postexposure prophylaxis against mpox.
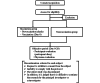
Methods: The study aimed to enroll 100 vaccinated participants who had been identified as close contacts of individuals with mpox. Consent was obtained, and the participants are inoculated with the vaccine. Daily recordings of symptoms (body temperature, headache, rash, and side effects) were made until day 21 and then again on day 28. Furthermore, additional evaluations of adverse events were performed by the investigators on days 7, 14, 21, and 28. Considering that the maximum incubation period for mpox is 21 days, the primary end point is the presence or absence of the disease 21 days after close contact. The primary analysis focused on cases within 4 days of intense contact as it has been reported that vaccination within this timeframe can reduce the incidence of the disease.
Results: The first trial participant was enrolled on July 28, 2022, and the research period concluded in March 2023. The study results will be published in a peer-reviewed scientific journal.
Conclusions: This study allowed us to investigate the efficacy and safety of the LC16m8 strain vaccine in postexposure prophylaxis against mpox.
Trial registration: Japan Registry of Clinical Trials jRCTs031220137; https://jrct.niph.go.jp/en-latest-detail/jRCTs031220137.
International registered report identifier (irrid): DERR1-10.2196/46955.
Keywords: Disease Control and Prevention; LC16; body temperature; endpoint analysis; epidemiology; headache; immunity; infectious disease; injection site; inoculation; lymphadenopathy; monkeypox; orthopoxvirus; post-exposure prophylaxis; poxvirus; rash; smallpox; smallpox vaccine; smallpox virus; vaccination; vaccine; vaccinia virus; virus; zoonosis.
©Rina Yano, Junko Terada-Hirashima, Yukari Uemura, Noriko Tomita, Yosuke Shimizu, Haruka Iwasaki, Nobumasa Okumura, Tetsuya Suzuki, Sho Saito, Mugen Ujiie, Wataru Sugiura, Norio Ohmagari. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 25.08.2023.
Conflict of interest statement
Conflicts of Interest: None declared.
Similar articles
-
An open-label, non-randomized study investigating the safety and efficacy of smallpox vaccine, LC16, as post-exposure prophylaxis for mpox.Hum Vaccin Immunother. 2023 Aug 1;19(2):2242219. doi: 10.1080/21645515.2023.2242219. Hum Vaccin Immunother. 2023. PMID: 37559375 Free PMC article. Clinical Trial.
-
Evaluating the Immunogenicity and Safety of a Smallpox Vaccine to Monkeypox in Healthy Japanese Adults: A Single-Arm Study.Life (Basel). 2023 Mar 14;13(3):787. doi: 10.3390/life13030787. Life (Basel). 2023. PMID: 36983942 Free PMC article.
-
Mpox Neutralizing Antibody Response to LC16m8 Vaccine in Healthy Adults.NEJM Evid. 2024 Mar;3(3):EVIDoa2300290. doi: 10.1056/EVIDoa2300290. Epub 2024 Feb 27. NEJM Evid. 2024. PMID: 38411447
-
Progress and prospects on vaccine development against monkeypox infection.Microb Pathog. 2023 Jul;180:106156. doi: 10.1016/j.micpath.2023.106156. Epub 2023 May 16. Microb Pathog. 2023. PMID: 37201635 Free PMC article. Review.
-
Mpox: Rapid Evidence Review.Am Fam Physician. 2023 Jul;108(1):78-83. Am Fam Physician. 2023. PMID: 37440743 Review.
Cited by
-
Recent Advances in Mpox Epidemic: Global Features and Vaccine Prevention Research.Vaccines (Basel). 2025 Apr 25;13(5):466. doi: 10.3390/vaccines13050466. Vaccines (Basel). 2025. PMID: 40432078 Free PMC article. Review.
-
Advancements in monkeypox vaccines development: a critical review of emerging technologies.Front Immunol. 2024 Oct 11;15:1456060. doi: 10.3389/fimmu.2024.1456060. eCollection 2024. Front Immunol. 2024. PMID: 39464881 Free PMC article. Review.
References
-
- Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129–157. doi: 10.2217/fvl.12.130. https://europepmc.org/abstract/MED/23626656 - DOI - PMC - PubMed
-
- WHO recommends new name for monkeypox disease. World Health Organization. 2022. [2023-07-21]. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-mon... .
-
- Petersen E, Kantele A, Koopmans M, Asogun D, Yinka-Ogunleye A, Ihekweazu C, Zumla A. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. https://www.sciencedirect.com/science/article/pii/S0891552019300170?via%... S0891-5520(19)30017-0 - DOI - PMC - PubMed
-
- Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, Snell LB, Murphy J, Potter J, Tuudah C, Sundramoorthi R, Abeywickrema M, Pley C, Naidu V, Nebbia G, Aarons E, Botgros A, Douthwaite ST, van Nispen Tot Pannerden C, Winslow H, Brown A, Chilton D, Nori A. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. https://www.bmj.com/content/378/bmj-2022-072410 - DOI - PMC - PubMed
-
- Lourie B, Bingham PG, Evans HH, Foster SO, Nakano JH, Herrmann KL. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa. Bull World Health Organ. 1972;46(5):633–639. https://europepmc.org/abstract/MED/4340223 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials