Osteopontin drives retinal ganglion cell resiliency in glaucomatous optic neuropathy
- PMID: 37624696
- PMCID: PMC10591811
- DOI: 10.1016/j.celrep.2023.113038
Osteopontin drives retinal ganglion cell resiliency in glaucomatous optic neuropathy
Abstract
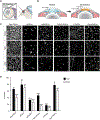
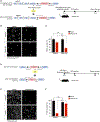
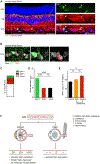
Chronic neurodegeneration and acute injuries lead to neuron losses via diverse processes. We compared retinal ganglion cell (RGC) responses between chronic glaucomatous conditions and the acute injury model. Among major RGC subclasses, αRGCs and intrinsically photosensitive RGCs (ipRGCs) preferentially survive glaucomatous conditions, similar to findings in the retina subject to axotomy. Focusing on an αRGC intrinsic factor, Osteopontin (secreted phosphoprotein 1 [Spp1]), we found an ectopic neuronal expression of Osteopontin (Spp1) in other RGCs subject to glaucomatous conditions. This contrasted with the Spp1 downregulation subject to axotomy. αRGC-specific Spp1 elimination led to significant αRGC loss, diminishing their resiliency. Spp1 overexpression led to robust neuroprotection of susceptible RGC subclasses under glaucomatous conditions. In contrast, Spp1 overexpression did not significantly protect RGCs subject to axotomy. Additionally, SPP1 marked adult human RGC subsets with large somata and SPP1 expression in the aqueous humor correlated with glaucoma severity. Our study reveals Spp1's role in mediating neuronal resiliency in glaucoma.
Keywords: CP: Neuroscience; Osteopontin; glaucoma; human retina; neuronal types; neuroprotection; optic nerve crush; retinal ganglion cell.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.S.W. is a founder of and consultant to Perceive Biotherapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

