Augmentation Therapy for Severe Alpha-1 Antitrypsin Deficiency Improves Survival and Is Decoupled from Spirometric Decline-A Multinational Registry Analysis
- PMID: 37624745
- PMCID: PMC10870866
- DOI: 10.1164/rccm.202305-0863OC
Augmentation Therapy for Severe Alpha-1 Antitrypsin Deficiency Improves Survival and Is Decoupled from Spirometric Decline-A Multinational Registry Analysis
Abstract
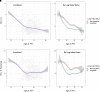
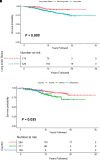
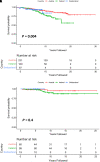
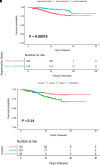
Rationale: Intravenous plasma-purified alpha-1 antitrypsin (IV-AAT) has been used as therapy for alpha-1 antitrypsin deficiency (AATD) since 1987. Previous trials (RAPID and RAPID-OLE) demonstrated efficacy in preserving computed tomography of lung density but no effect on FEV1. This observational study evaluated 615 people with severe AATD from three countries with socialized health care (Ireland, Switzerland, and Austria), where access to standard medical care was equal but access to IV-AAT was not. Objectives: To assess the real-world longitudinal effects of IV-AAT. Methods: Pulmonary function and mortality data were utilized to perform longitudinal analyses on registry participants with severe AATD. Measurements and Main Results: IV-AAT confers a survival benefit in severe AATD (P < 0.001). We uncovered two distinct AATD phenotypes based on an initial respiratory diagnosis: lung index and non-lung index. Lung indexes demonstrated a more rapid FEV1 decline between the ages of 20 and 50 and subsequently entered a plateau phase of minimal decline from 50 onward. Consequentially, IV-AAT had no effect on FEV1 decline, except in patients with a Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 lung index. Conclusions: This real-world study demonstrates a survival advantage from IV-AAT. This improved survival is largely decoupled from FEV1 decline. The observation that patients with severe AATD fall into two major phenotypes has implications for clinical trial design where FEV1 is a primary endpoint. Recruits into trials are typically older lung indexes entering the plateau phase and, therefore, unlikely to show spirometric benefits. IV-AAT attenuates spirometric decline in lung indexes in GOLD stage 2, a spirometric group commonly outside current IV-AAT commencement recommendations.
Keywords: Kaplan-Meier survival curve; forced expiratory volume; universal health care; α 1-proteinase inhibitor.
Figures
Comment in
-
Treatment for Alpha-1 Antitrypsin Deficiency: Does Augmentation Therapy Work?Am J Respir Crit Care Med. 2023 Nov 1;208(9):948-949. doi: 10.1164/rccm.202309-1585ED. Am J Respir Crit Care Med. 2023. PMID: 37724887 Free PMC article. No abstract available.
-
Identification of Alpha-1 Antitrypsin-Deficient Subjects with Normal Spirometry Who May Benefit from Alpha-1 Antitrypsin Replacement.Am J Respir Crit Care Med. 2024 Apr 15;209(8):1033-1034. doi: 10.1164/rccm.202311-2189LE. Am J Respir Crit Care Med. 2024. PMID: 38237158 Free PMC article. No abstract available.
-
A1AT-Mangel: Substitution senkt Mortalität.MMW Fortschr Med. 2024 Oct;166(18):28-30. doi: 10.1007/s15006-024-4402-5. MMW Fortschr Med. 2024. PMID: 39448478 Review. German. No abstract available.
References
-
- Laurell C-B, Eriksson S. The electrophoretic α; 1-globulin pattern of serum in α; 1-antitrypsin deficiency. Scand J Clin Lab Invest . 1963;15:132–140.
-
- Ganrot PO, Laurell C-B, Eriksson S. Obstructive lung disease and trypsin inhibitors in α-1-antitrypsin deficiency. Scand J Clin Lab Invest . 1967;19:205–208. - PubMed
-
- Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med . 1987;316:1055–1062. - PubMed
-
- Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DC, Ulrik CS, et al. A randomized clinical trial of α(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med . 1999;160:1468–1472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

