The iron chaperone poly(rC)-binding protein 1 regulates iron efflux through intestinal ferroportin in mice
- PMID: 37624904
- PMCID: PMC10656723
- DOI: 10.1182/blood.2023020504
The iron chaperone poly(rC)-binding protein 1 regulates iron efflux through intestinal ferroportin in mice
Abstract
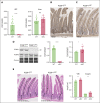
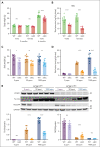
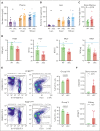
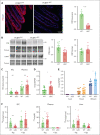
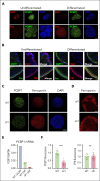
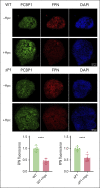
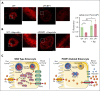
Iron is an essential nutrient required by all cells but used primarily for red blood cell production. Because humans have no effective mechanism for ridding the body of excess iron, the absorption of dietary iron must be precisely regulated. The critical site of regulation is the transfer of iron from the absorptive enterocyte to the portal circulation via the sole iron efflux transporter, ferroportin. Here, we report that poly(rC)-binding protein 1 (PCBP1), the major cytosolic iron chaperone, is necessary for the regulation of iron flux through ferroportin in the intestine of mice. Mice lacking PCBP1 in the intestinal epithelium exhibit low levels of enterocyte iron, poor retention of dietary iron in enterocyte ferritin, and excess efflux of iron through ferroportin. Excess iron efflux occurred despite lower levels of ferroportin protein in enterocytes and upregulation of the iron regulatory hormone hepcidin. PCBP1 deletion and the resulting unregulated dietary iron absorption led to poor growth, severe anemia on a low-iron diet, and liver oxidative stress with iron loading on a high-iron diet. Ex vivo culture of PCBP1-depleted enteroids demonstrated no defects in hepcidin-mediated ferroportin turnover. However, measurement of kinetically labile iron pools in enteroids competent or blocked for iron efflux indicated that PCBP1 functioned to bind and retain cytosolic iron and limit its availability for ferroportin-mediated efflux. Thus, PCBP1 coordinates enterocyte iron and reduces the concentration of unchaperoned "free" iron to a low level that is necessary for hepcidin-mediated regulation of ferroportin activity.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.D.H. is Eurofins Lancaster Laboratories, Inc, Lancaster, PA.
The current affiliation for M.S.-E. is Laboratory of Cellular and Developmental Biology, National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD.
Figures


Comment in
-
PCBP1 is essential for proper iron absorption.Blood. 2023 Nov 9;142(19):1585-1587. doi: 10.1182/blood.2023022267. Blood. 2023. PMID: 37944181 Free PMC article. No abstract available.
Similar articles
-
Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2.J Biol Chem. 2016 Aug 12;291(33):17303-18. doi: 10.1074/jbc.M116.721936. Epub 2016 Jun 14. J Biol Chem. 2016. PMID: 27302059 Free PMC article.
-
The relevance of the intestinal crypt and enterocyte in regulating iron absorption.Pflugers Arch. 2007 Nov;455(2):201-13. doi: 10.1007/s00424-007-0264-9. Epub 2007 May 1. Pflugers Arch. 2007. PMID: 17473933 Review.
-
Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C450-9. doi: 10.1152/ajpcell.00348.2013. Epub 2013 Dec 4. Am J Physiol Cell Physiol. 2014. PMID: 24304836 Free PMC article.
-
The role of hepcidin and ferroportin in iron absorption.Histol Histopathol. 2007 Jul;22(7):791-804. doi: 10.14670/HH-22.791. Histol Histopathol. 2007. PMID: 17455153 Review.
-
Current understanding of iron homeostasis.Am J Clin Nutr. 2017 Dec;106(Suppl 6):1559S-1566S. doi: 10.3945/ajcn.117.155804. Epub 2017 Oct 25. Am J Clin Nutr. 2017. PMID: 29070551 Free PMC article. Review.
Cited by
-
PCBP1 is essential for proper iron absorption.Blood. 2023 Nov 9;142(19):1585-1587. doi: 10.1182/blood.2023022267. Blood. 2023. PMID: 37944181 Free PMC article. No abstract available.
-
HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease.Biomedicines. 2025 Mar 10;13(3):683. doi: 10.3390/biomedicines13030683. Biomedicines. 2025. PMID: 40149659 Free PMC article. Review.
-
Dietary Iron Absorption: Biochemical and Nutritional Aspects.Adv Exp Med Biol. 2025;1480:75-87. doi: 10.1007/978-3-031-92033-2_6. Adv Exp Med Biol. 2025. PMID: 40603785 Review.
-
Iron Absorption: Molecular and Pathophysiological Aspects.Metabolites. 2024 Apr 17;14(4):228. doi: 10.3390/metabo14040228. Metabolites. 2024. PMID: 38668356 Free PMC article. Review.
-
Acevaltrate as a novel ferroptosis inducer with dual targets of PCBP1/2 and GPX4 in colorectal cancer.Signal Transduct Target Ther. 2025 Jul 7;10(1):211. doi: 10.1038/s41392-025-02296-7. Signal Transduct Target Ther. 2025. PMID: 40619432 Free PMC article.
References
-
- Katsarou A, Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol Aspects Med. 2020;75 - PubMed
-
- Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397(10270):233–248. - PubMed
-
- Yanatori I, Kishi F. DMT1 and iron transport. Free Radic Biol Med. 2019;133:55–63. - PubMed
-
- Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

