The oxylipin analog CS585 prevents platelet activation and thrombosis through activation of the prostacyclin receptor
- PMID: 37624927
- PMCID: PMC10656727
- DOI: 10.1182/blood.2023020622
The oxylipin analog CS585 prevents platelet activation and thrombosis through activation of the prostacyclin receptor
Abstract
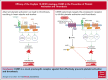
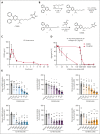
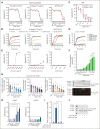
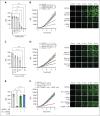
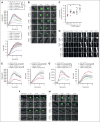
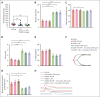
Cardiovascular disease remains the primary cause of morbidity and mortality globally. Platelet activation is critical for maintaining hemostasis and preventing the leakage of blood cells from the vessel. There has been a paucity in the development of new drugs to target platelet reactivity. Recently, the oxylipin 12(S)-hydroxy-eicosatrienoic acid (12-HETrE), which is produced in platelets, was shown to limit platelet reactivity by activating the prostacyclin receptor. Here, we demonstrated the synthesis of a novel analog of 12-HETrE, known as CS585. Human blood and mouse models of hemostasis and thrombosis were assessed for the ability of CS585 to attenuate platelet activation and thrombosis without increasing the risk of bleeding. Human platelet activation was assessed using aggregometry, flow cytometry, western blot analysis, total thrombus formation analysis system, microfluidic perfusion chamber, and thromboelastography. Hemostasis, thrombosis, and bleeding assays were performed in mice. CS585 was shown to potently target the prostacyclin receptor on the human platelet, resulting in a highly selective and effective mechanism for the prevention of platelet activation. Furthermore, CS585 was shown to inhibit platelet function in human whole blood ex vivo, prevent thrombosis in both small and large vessels in mouse models, and exhibit long-lasting prevention of clot formation. Finally, CS585 was not observed to perturb coagulation or increase the risk of bleeding in the mouse model. Hence, CS585 represents a new validated target for the treatment of thrombotic diseases without the risk of bleeding or off-target activation observed with other prostaglandin receptor agonists.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.H. is an equity holder and serves on the scientific advisory board for Veralox Therapeutics; is an equity holder and consultant for Cereno Scientific; and has received research funding from Cereno Scientific for this project. Additionally, M.H. and A.W. are listed as inventors of CS585 with associated patents US11,236,044 and US11,498,905. B.D. is the Chief Medical Officer for Cereno Scientific and is an equity holder. N.B. serves as a scientific adviser for Cereno Scientific and is an equity holder. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Targeting prostacyclin: all gain with no pain?Blood. 2023 Nov 2;142(18):1506-1507. doi: 10.1182/blood.2023022227. Blood. 2023. PMID: 37917084 No abstract available.
References
-
- Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19(5):333–352. - PubMed
-
- Adamski P, Koziński M, Ostrowska M, et al. Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb Haemost. 2014;112(2):224–242. - PubMed
-
- Ostrowska M, Adamski P, Koziński M, et al. Off-target effects of glycoprotein IIb/IIIa receptor inhibitors. Cardiol J. 2014;21(5):458–464. - PubMed
-
- Serebruany VL, Fortmann SD, Rao SV, et al. Vorapaxar and diplopia: possible off-target PAR-receptor mismodulation. Thromb Haemost. 2016;115(5):905–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

