Engineered Biocatalytic Synthesis of β-N-Substituted-α-Amino Acids
- PMID: 37625129
- PMCID: PMC10592029
- DOI: 10.1002/anie.202311189
Engineered Biocatalytic Synthesis of β-N-Substituted-α-Amino Acids
Abstract
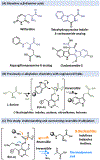
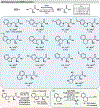
Non-canonical amino acids (ncAAs) are useful synthons for the development of new medicines, materials, and probes for bioactivity. Recently, enzyme engineering has been leveraged to produce a suite of highly active enzymes for the synthesis of β-substituted amino acids. However, there are few examples of biocatalytic N-substitution reactions to make α,β-diamino acids. In this study, we used directed evolution to engineer the β-subunit of tryptophan synthase, TrpB, for improved activity with diverse amine nucleophiles. Mechanistic analysis shows that high yields are hindered by product re-entry into the catalytic cycle and subsequent decomposition. Additional equivalents of l-serine can inhibit product reentry through kinetic competition, facilitating preparative scale synthesis. We show β-substitution with a dozen aryl amine nucleophiles, including demonstration on a gram scale. These transformations yield an underexplored class of amino acids that can serve as unique building blocks for chemical biology and medicinal chemistry.
Keywords: Biocatalysis; Directed Evolution; Multiplexed Screening; Non-Canonical Amino Acids; Pyridoxal Phosphate.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures



References
-
- Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM, Barreca D, Weckwerth W, Bauer R, Bayer EA, Majeed M, Bishayee A, Bochkov V, Bonn GK, Braidy N, Bucar F, Cifuentes A, D’Onofrio G, Bodkin M, Diederich M, Dinkova-Kostova AT, Efferth T, el Bairi K, Arkells N, Fan TP, Fiebich BL, Freissmuth M, Georgiev MI, Gibbons S, Godfrey KM, Gruber CW, Heer J, Huber LA, Ibanez E, Kijjoa A, Kiss AK, Lu A, Macias FA, Miller MJS, Mocan A, Müller R, Nicoletti F, Perry G, Pittalà V, Rastrelli L, Ristow M, Russo GL, Silva AS, Schuster D, Sheridan H, Skalicka-Woźniak K, Skaltsounis L, Sobarzo-Sánchez E, Bredt DS, Stuppner H, Sureda A, Tzvetkov NT, Vacca RA, Aggarwal BB, Battino M, Giampieri F, Wink M, Wolfender JL, Xiao J, Yeung AWK, Lizard G, Popp MA, Heinrich M, Berindan-Neagoe I, Stadler M, Daglia M, Verpoorte R, Supuran CT, Nature Reviews Drug Discovery 2021 20:3 2021, 20, 200–216. - PMC - PubMed
-
- Zhao H, Liu C, Ding W, Tang L, Fang Y, Chen Y, Hu L, Yuan Y, Fang D, Lin S, J Am Chem Soc 2022, 144, 6742–6748. - PubMed
-
- Budisa N, Engineering the Genetic Code: Expanding the Amino Acid Repertoire for the Design of Novel Proteins, Wiley-VCH, Weinheim, 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

