Long-acting lenacapavir protects macaques against intravenous challenge with simian-tropic HIV
- PMID: 37625266
- PMCID: PMC10470178
- DOI: 10.1016/j.ebiom.2023.104764
Long-acting lenacapavir protects macaques against intravenous challenge with simian-tropic HIV
Abstract
Background: Long-acting subcutaneous lenacapavir (LEN), a first-in-class HIV capsid inhibitor approved by the US FDA for the treatment of multidrug-resistant HIV-1 with twice yearly dosing, is under investigation for HIV-1 pre-exposure prophylaxis (PrEP). We previously derived a simian-tropic HIV-1 clone (stHIV-A19) that encodes an HIV-1 capsid and replicates to high titres in pigtail macaques (PTM), resulting in a nonhuman primate model well-suited for evaluating LEN PrEP in vivo.
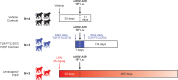
Methods: Lenacapavir potency against stHIV-A19 in PTM peripheral blood mononuclear cells in vitro was determined and subcutaneous LEN pharmacokinetics were evaluated in naïve PTMs in vivo. To evaluate the protective efficacy of LEN PrEP, naïve PTMs received either a single subcutaneous injection of LEN (25 mg/kg, N = 3) or vehicle (N = 4) 30 days before a high-dose intravenous challenge with stHIV-A19, or 7 daily subcutaneous injections of a 3-drug control PrEP regimen starting 3 days before stHIV-A19 challenge (N = 3).
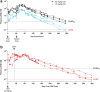
Findings: In vitro, LEN showed potent antiviral activity against stHIV-A19, comparable to its potency against HIV-1. In vivo, subcutaneous LEN displayed sustained plasma drug exposures in PTMs. Following stHIV-A19 challenge, while all vehicle control animals became productively infected, all LEN and 3-drug control PrEP animals were protected from infection.
Interpretation: These findings highlight the utility of the stHIV-A19/PTM model and support the clinical development of long-acting LEN for PrEP in humans.
Funding: Gilead Sciences as part of a Cooperative Research and Development Agreement between Gilead Sciences and Frederick National Lab; federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024/HHSN261201500003I; NIH grant R01AI078788.
Keywords: Capsid; HIV; Macaque; Nonhuman primate; PrEP; Pre-exposure prophylaxis.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests BL, KW, WR, JZ, WB, and SRY are or were employees of Gilead Sciences, Inc., and received salary and stock ownership as compensation for their employment. WB, JZ, and SRY are inventors on patent application US20210188815A1 covering the use of capsid inhibitors for the prevention of HIV. JDL has served as a compensated advisor to Gilead Sciences, Inc., and JDL, GQD, and AES have received research support from Gilead Sciences, Inc., for studies unrelated to the work described here through separate collaborative research and development agreements with Leidos Biomedical Research, Inc. TH has received royalties from Wiley for textbook authorship, NIH honoraria for grant review panel participation, and registration fee reimbursement from Gordon conference for participating as an invited speaker. PDB receives salary support from the Howard Hughes Medical Institute and owns Gilead stock. The authors declare no other financial or competing interests exist.
Figures





References
-
- UNAIDS . 2021. Global HIV & AIDS statistics - 2021 fact sheet.http://wwwunaidsorg/en/resources/fact-sheet
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

