Durable alveolar engraftment of PSC-derived lung epithelial cells into immunocompetent mice
- PMID: 37625412
- PMCID: PMC10529386
- DOI: 10.1016/j.stem.2023.07.016
Durable alveolar engraftment of PSC-derived lung epithelial cells into immunocompetent mice
Abstract
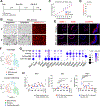
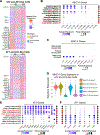
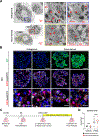
Durable reconstitution of the distal lung epithelium with pluripotent stem cell (PSC) derivatives, if realized, would represent a promising therapy for diseases that result from alveolar damage. Here, we differentiate murine PSCs into self-renewing lung epithelial progenitors able to engraft into the injured distal lung epithelium of immunocompetent, syngeneic mouse recipients. After transplantation, these progenitors mature in the distal lung, assuming the molecular phenotypes of alveolar type 2 (AT2) and type 1 (AT1) cells. After months in vivo, donor-derived cells retain their mature phenotypes, as characterized by single-cell RNA sequencing (scRNA-seq), histologic profiling, and functional assessment that demonstrates continued capacity of the engrafted cells to proliferate and differentiate. These results indicate durable reconstitution of the distal lung's facultative progenitor and differentiated epithelial cell compartments with PSC-derived cells, thus establishing a novel model for pulmonary cell therapy that can be utilized to better understand the mechanisms and utility of engraftment.
Keywords: alveolar epithelium; bleomycin injury; directed differentiation; distal lung bud tip cells; lung; lung progenitors; pluripotent stem cells; stem cells.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman J-P, Davis JL, Heilwell G, Spirn M, et al. (2015). Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516. 10.1016/s0140-6736(14)61376-3. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

