Characterization of Rab32- and Rab38-positive lysosome-related organelles in osteoclasts and macrophages
- PMID: 37625588
- PMCID: PMC10518718
- DOI: 10.1016/j.jbc.2023.105191
Characterization of Rab32- and Rab38-positive lysosome-related organelles in osteoclasts and macrophages
Abstract
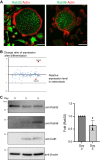
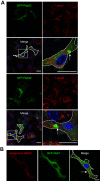
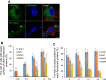
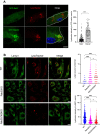
Both the biogenesis and functions of osteoclasts and macrophages involves dynamic membrane traffic. We screened transcript levels for Rab family small GTPases related to osteoclasts and identified Rab38. Rab38 expression is upregulated during osteoclast differentiation and maturation. In osteoclasts, both Rab38 and its paralog, Rab32, colocalize to lysosome-related organelles (LROs). In macrophages, Rab32 is also found in LROs. LROs are part of the endocytic pathway but are distinct from lysosomes. After receptor activator of NF-κB ligand stimulation, LROs contain cathepsin K and tartrate-resistant acid phosphatase inside and help both proteins to accumulate around bone resorption pits. After osteoclast maturation, these enzymes are hardly found within LROs. In macrophages derived from Rab32 and Rab38 double knockout mice, both acidification and V-ATPase a3 localization were severely compromised. Both the double knockout macrophage and bafilomycin-treated wildtype macrophage show an increase in Lamp1-positive organelles, implying that biogenesis of lysosomes and LROs are related. These results indicate that Rab32 and Rab38 both play a crucial role in LRO biogenesis in macrophages and in osteoclasts.
Keywords: Rab32; Rab38; lysosome-related organelles; macrophage; osteoclast.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest with the contents of this article.
Figures




References
-
- Nakashima T., Hayashi M., Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012;23:582–590. - PubMed
-
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. - PubMed
-
- Jurdic P., Saltel F., Chabadel A., Destaing O. Podosome and sealing zone: Specificity of the osteoclast model. Eur. J. Cell Biol. 2006;85:195–202. - PubMed
-
- Stenbeck G. Formation and function of the ruffled border in osteoclasts. Semin. Cell Dev. Biol. 2002;13:285–292. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

