Targeted deletion of von-Hippel-Lindau in the proximal tubule conditions the kidney against early diabetic kidney disease
- PMID: 37626062
- PMCID: PMC10457389
- DOI: 10.1038/s41419-023-06074-7
Targeted deletion of von-Hippel-Lindau in the proximal tubule conditions the kidney against early diabetic kidney disease
Abstract
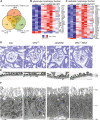
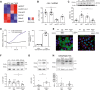
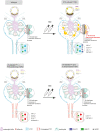
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease. Glomerular hyperfiltration and albuminuria subject the proximal tubule (PT) to a subsequent elevation of workload, growth, and hypoxia. Hypoxia plays an ambiguous role in the development and progression of DKD and shall be clarified in our study. PT-von-Hippel-Lindau (Vhl)-deleted mouse model in combination with streptozotocin (STZ)-induced type I diabetes mellitus (DM) was phenotyped. In contrary to PT-Vhl-deleted STZ-induced type 1 DM mice, proteinuria and glomerular hyperfiltration occurred in diabetic control mice the latter due to higher nitric oxide synthase 1 and sodium and glucose transporter expression. PT Vhl deletion and DKD share common alterations in gene expression profiles, including glomerular and tubular morphology, and tubular transport and metabolism. Compared to diabetic control mice, the most significantly altered in PT Vhl-deleted STZ-induced type 1 DM mice were Ldc-1, regulating cellular oxygen consumption rate, and Zbtb16, inhibiting autophagy. Alignment of altered genes in heat maps uncovered that Vhl deletion prior to STZ-induced DM preconditioned the kidney against DKD. HIF-1α stabilization leading to histone modification and chromatin remodeling resets most genes altered upon DKD towards the control level. These data demonstrate that PT HIF-1α stabilization is a hallmark of early DKD and that targeting hypoxia prior to the onset of type 1 DM normalizes renal cell homeostasis and prevents DKD development.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17. doi: 10.1016/S2213-8587(19)30180-9. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

