Maintenance of pig brain function under extracorporeal pulsatile circulatory control (EPCC)
- PMID: 37626089
- PMCID: PMC10457326
- DOI: 10.1038/s41598-023-39344-7
Maintenance of pig brain function under extracorporeal pulsatile circulatory control (EPCC)
Abstract
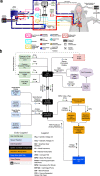
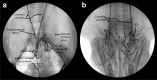
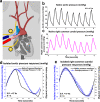
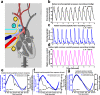
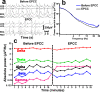
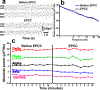
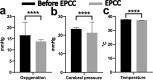
Selective vascular access to the brain is desirable in metabolic tracer, pharmacological and other studies aimed to characterize neural properties in isolation from somatic influences from chest, abdomen or limbs. However, current methods for artificial control of cerebral circulation can abolish pulsatility-dependent vascular signaling or neural network phenomena such as the electrocorticogram even while preserving individual neuronal activity. Thus, we set out to mechanically render cerebral hemodynamics fully regulable to replicate or modify native pig brain perfusion. To this end, blood flow to the head was surgically separated from the systemic circulation and full extracorporeal pulsatile circulatory control (EPCC) was delivered via a modified aorta or brachiocephalic artery. This control relied on a computerized algorithm that maintained, for several hours, blood pressure, flow and pulsatility at near-native values individually measured before EPCC. Continuous electrocorticography and brain depth electrode recordings were used to evaluate brain activity relative to the standard offered by awake human electrocorticography. Under EPCC, this activity remained unaltered or minimally perturbed compared to the native circulation state, as did cerebral oxygenation, pressure, temperature and microscopic structure. Thus, our approach enables the study of neural activity and its circulatory manipulation in independence of most of the rest of the organism.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

