Molecular surveillance of Plasmodium falciparum drug-resistance markers in Vietnam using multiplex amplicon sequencing (2000-2016)
- PMID: 37626131
- PMCID: PMC10457381
- DOI: 10.1038/s41598-023-40935-7
Molecular surveillance of Plasmodium falciparum drug-resistance markers in Vietnam using multiplex amplicon sequencing (2000-2016)
Erratum in
-
Author Correction: Molecular surveillance of Plasmodium falciparum drug-resistance markers in Vietnam using multiplex amplicon sequencing (2000-2016).Sci Rep. 2023 Oct 11;13(1):17207. doi: 10.1038/s41598-023-43996-w. Sci Rep. 2023. PMID: 37821479 Free PMC article. No abstract available.
Abstract
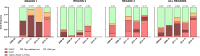
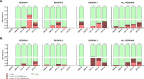
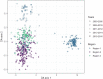
Emergence and spread of Plasmodium falciparum resistance to artemisinin-based combination therapies (ACT) is a major challenge for Greater Mekong Subregion countries in their goal to eliminate malaria by 2030. Tools to efficiently monitor drug resistance beyond resource-demanding therapeutic efficacy studies are necessary. A custom multiplex amplicon sequencing assay based on Illumina technology was designed to target the marker of partial resistance to artemisinin (K13), five candidate modulators of artemisinin resistance, the marker of resistance to chloroquine (crt), and four neutral microsatellite loci. The assay was used to genotype 635 P. falciparum-positive blood samples collected across seven provinces of Vietnam and one of Cambodia between 2000 and 2016. Markers of resistance to artemisinin partner-drugs piperaquine (copy number of plasmepsin-2) and mefloquine (copy number of multidrug-resistance 1) were determined by qPCR. Parasite population structure was further assessed using a 101-SNP barcode. Validated mutations of artemisinin partial resistance in K13 were found in 48.1% of samples, first detection was in 2000, and by 2015 prevalence overcame > 50% in Central Highlands and Binh Phuoc province. K13-C580Y variant became predominant country-wide, quickly replacing an outbreak of K13-I543T in Central Highlands. Mutations in candidate artemisinin resistance modulator genes paralleled the trends of K13 mutants, whereas resistance to piperaquine and mefloquine remained low (≈ 10%) by 2015-2016. Genomic tools applied to malaria surveillance generate comprehensive information on dynamics of drug resistance and population structure and reflect drug efficacy profiles from in vivo studies.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


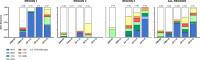
References
-
- World Health Organization. Guidelines for malaria. Global Malaria Program (2021).
-
- World Health Organization. Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019) (2020).
-
- World Health Organization. Methods for Surveillance of Antimalarial Drug Efficacy (2009).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

