VSIG2 promotes malignant progression of pancreatic ductal adenocarcinoma by enhancing LAMTOR2-mediated mTOR activation
- PMID: 37626304
- PMCID: PMC10463957
- DOI: 10.1186/s12964-023-01209-x
VSIG2 promotes malignant progression of pancreatic ductal adenocarcinoma by enhancing LAMTOR2-mediated mTOR activation
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is one of the most intractable malignancies to overcome clinically due to its insidious onset as well as rapid progression. It is urgent to seek new diagnostic markers and therapeutic targets in order to furthest ameliorate the prognosis of patients with PDAC. V-set and immunoglobulin domain containing 2 (VSIG2) belongs to immunoglobulin superfamily (IgSF), which function as coinhibitory molecule to mediate immune evasion of tumors. Nevertheless, the role of VSIG2 in PDAC and related mechanism still keep unclear.
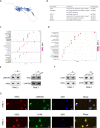
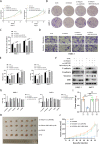
Methods: Different expression of VSIG2 in PDAC tissues and cells were detected by bioinformatic analysis, immunohistochemistry, real-time quantitative PCR as well as western blotting. CCK-8, colony formation, Transwell assay, and scratch experiment were utilized to assess proliferation, invasion and migration properties of PDAC cells. The relationship of VSIG2 with late endosomal/lysosomal adaptor, MAPK and MTOR activator 2 (LAMTOR2) and mechanistic target of rapamycin (mTOR) was identified using mass spectrometry, co-immunoprecipitation and immunofluorescence. GO and KEGG enrichment analysis were performed for further pathway verification using western blotting. Additionally, subcutaneous xenograft tumor model and clinical samples analysis were implemented to further elucidate the oncogenic effect of VSIG2 on PDAC in vivo and clinically.
Results: VSIG2 was highly expressed in PDAC tissues and cells. Overexpression of VSIG2 facilitated the proliferation, invasion and migration abilities of PDAC cells, while VSIG2-inhibition exerted opposite effects. Mechanistically, VSIG2 could simultaneously bind to LAMTOR2 and mTOR, thereby enhancing interaction between two molecules, which resulted in elevated phosphorylation-modificatory activation of mTOR and downstream key molecules. Clinically, up-regulation of VSIG2 was positively associated with advanced stage, overall survival and disease-free survival of PDAC patients.
Conclusions: Our study disclosed that VSIG2 was overexpressed in PDAC, which promoted the proliferation, invasion and metastasis. Mechanically, VSIG2 acted as a scaffold to recruit LAMTOR2 and mTOR simultaneously, stabilize the interaction between them, thus enhancing LAMTOR2-mediated mTOR phosphorylated activation. Collectively, VSIG2 could be exploited as a biomarker for diagnosis and prognosis monitor of PDAC in the future, meanwhile, targeting VSIG2 in PDAC management is expected to be a novel strategy. Video Abstract. Video Abstract.
Keywords: Late endosomal/lysosomal adaptor; MAPK and MTOR activator 2 (LAMTOR2); Malignant progression; Mechanistic target of rapamycin (mTOR); Pancreatic ductal adenocarcinoma; V-set and immunoglobulin domain containing 2 (VSIG2).
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

