A study on the effect of natural products against the transmission of B.1.1.529 Omicron
- PMID: 37626376
- PMCID: PMC10464336
- DOI: 10.1186/s12985-023-02160-6
A study on the effect of natural products against the transmission of B.1.1.529 Omicron
Abstract
Background: The recent outbreak of the Coronavirus pandemic resulted in a successful vaccination program launched by the World Health Organization. However, a large population is still unvaccinated, leading to the emergence of mutated strains like alpha, beta, delta, and B.1.1.529 (Omicron). Recent reports from the World Health Organization raised concerns about the Omicron variant, which emerged in South Africa during a surge in COVID-19 cases in November 2021. Vaccines are not proven completely effective or safe against Omicron, leading to clinical trials for combating infection by the mutated virus. The absence of suitable pharmaceuticals has led scientists and clinicians to search for alternative and supplementary therapies, including dietary patterns, to reduce the effect of mutated strains.
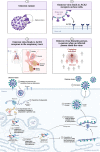
Main body: This review analyzed Coronavirus aetiology, epidemiology, and natural products for combating Omicron. Although the literature search did not include keywords related to in silico or computational research, in silico investigations were emphasized in this study. Molecular docking was implemented to compare the interaction between natural products and Chloroquine with the ACE2 receptor protein amino acid residues of Omicron. The global Omicron infection proceeding SARS-CoV-2 vaccination was also elucidated. The docking results suggest that DGCG may bind to the ACE2 receptor three times more effectively than standard chloroquine.
Conclusion: The emergence of the Omicron variant has highlighted the need for alternative therapies to reduce the impact of mutated strains. The current review suggests that natural products such as DGCG may be effective in binding to the ACE2 receptor and combating the Omicron variant, however, further research is required to validate the results of this study and explore the potential of natural products to mitigate COVID-19.
Keywords: COVID-19; In silico; Molecular docking; Natural product; Omicron; Vaccine.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures













References
-
- Organization WH. Enhancing response to Omicron SARS-CoV-2 variant: Technical brief and priority actions for Member States. World Health Organization Headquarters, Geneva, Switzerland Update 2022.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

