Antibody response to SARS-CoV-2 vaccines in patients with relapsing multiple sclerosis treated with evobrutinib: A Bruton's tyrosine kinase inhibitor
- PMID: 37626477
- PMCID: PMC10580670
- DOI: 10.1177/13524585231192460
Antibody response to SARS-CoV-2 vaccines in patients with relapsing multiple sclerosis treated with evobrutinib: A Bruton's tyrosine kinase inhibitor
Abstract
Background: Evobrutinib is an oral, central nervous system (CNS)-penetrant and highly selective covalent Bruton's tyrosine kinase inhibitor under clinical development for patients with relapsing multiple sclerosis (RMS).
Objective: To investigate the effect of evobrutinib on immune responses in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinated patients with RMS from a Phase II trial (NCT02975349).
Methods: A post hoc analysis of patients with RMS who received evobrutinib 75 mg twice daily and SARS-CoV-2 vaccines during the open-label extension (n = 45) was conducted. Immunoglobulin (Ig)G anti-S1/S2-specific SARS-CoV-2 antibodies were measured using an indirect chemiluminescence immunoassay.
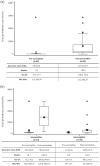
Results: In the vaccinated subgroup, mean/minimum evobrutinib exposure pre-vaccination was 105.2/88.7 weeks. In total, 43 of 45 patients developed/increased S1/S2 IgG antibody levels post-vaccination; one patient's antibody response remained negative post-vaccination and the other had antibody levels above the upper limit of detection, both pre- and post-vaccination. Most patients (n = 36/45), regardless of pre-vaccination serostatus, had a 10-100-fold increase of antibody levels pre- to post-vaccination. Antibody levels post-booster were higher versus post-vaccination.
Conclusion: These results suggest evobrutinib, an investigational drug with therapeutic potential for patients with RMS, acts as an immunomodulator, that is, it inhibits aberrant immune cell responses in patients with RMS, while responsiveness to foreign de novo and recall antigens is maintained.
Keywords: Bruton’s tyrosine kinase; COVID-19; Evobrutinib; SARS-CoV-2; multiple sclerosis; vaccines.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A Bar-Or holds the Melissa and Paul Anderson Chair. He has participated as a speaker in meetings sponsored by and received consulting fees from Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck Healthcare KGaA, Darmstadt, Germany, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Novartis, Roche/Genentech and Sanofi-Genzyme. He has received grant support to the University of Pennsylvania from Biogen Idec, Merck Healthcare KGaA, Darmstadt, Germany, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Novartis and Roche/Genentech.AH Cross has received consulting fees, research support and honoraria from Biogen, Bristol-Myers Squibb, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Merck Healthcare KGaA, Darmstadt, Germany, Genentech, Roche, Horizon, Janssen (subsidiary of Johnson & Johnson), Novartis, OCTAVE, TG Therapeutics, Academic CME, and WebMD; serves on the scientific advisory boards for ASCLEPIOS I/II for Novartis, and EVOLUTION I/II for EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA; has received grants from the United States Department of Defense; is President of the Board of Governors of the Consortium of Multiple Sclerosis Centers; and is a member of the advisory board of the International Progressive MS Alliance.AL Cunningham has received consultant fees to his institution from GSK, Seqirus and EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA.Y Hyvert, A Seitzinger, N Alexandri and H Gühring are employees of Merck Healthcare KGaA, Darmstadt, Germany.EE Drouin is an employee of EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaAD Tomic is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany, and received stock or an ownership interest from Novartis.X Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Bayer, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Darmstadt, Germany, Genzyme, F. Hoffmann-La Roche Ltd., Immunic, Janssen Pharmaceuticals, Medday, Merck KGaA, Darmstadt, Germany, Mylan, Nervgen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF and NMSS.
Figures


References
-
- Filippi M, Bar-Or A, Piehl F, et al.. Multiple sclerosis. Nat Rev Dis Prim 2018; 4: 43. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

