Subacute Partially Reversible Leukoencephalopathy Expands the Aicardi-Goutières Syndrome Phenotype
- PMID: 37626525
- PMCID: PMC10452434
- DOI: 10.3390/brainsci13081169
Subacute Partially Reversible Leukoencephalopathy Expands the Aicardi-Goutières Syndrome Phenotype
Abstract
Objective: To report a series of atypical presentations of Aicardi-Goutières syndrome.
Methods: Clinical, neuroimaging, and genetic data.
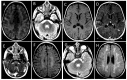
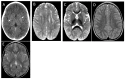
Results: We report a series of six unrelated patients (five males) with a subacute loss of developmental milestones, pyramidal signs, and regression of communication abilities, with onset at ages ranging from 7 to 20 months, reaching a nadir after 4 to 24 weeks. A remarkable improvement of lost abilities occurred in the follow-up, and they remained with residual spasticity and dysarthria but preserved cognitive function. Immunization or febrile illness occurred before disease onset in all patients. CSF was normal in two patients, and in four, borderline or mild lymphocytosis was present. A brain CT scan disclosed a subtle basal ganglia calcification in one of six patients. Brain MRI showed asymmetric signal abnormalities of white matter with centrum semi-ovale involvement in five patients and a diffuse white matter abnormality with contrast enhancement in one. Four patients were diagnosed and treated for acute demyelinating encephalomyelitis (ADEM). Brain imaging was markedly improved with one year or more of follow-up (average of 7 years), but patients remained with residual spasticity and dysarthria without cognitive impairment. Demyelination relapse occurred in a single patient four years after the first event. Whole-exome sequencing (WES) was performed in all patients: four of them disclosed biallelic pathogenic variants in RNASEH2B (three homozygous p.Ala177Thr and one compound heterozygous p.Ala177Thr/p.Gln58*) and in two of them the same homozygous deleterious variants in RNASEH2A (p.Ala249Val).
Conclusions: This report expands the phenotype of AGS to include subacute developmental regression with partial clinical and neuroimaging improvement. Those clinical features might be misdiagnosed as ADEM.
Keywords: Aicardi–Goutières syndrome; acute demyelinating encephalomyelitis; reversible leukoencephalopathy.
Conflict of interest statement
Fabiola P. Monteiro: former employee of Mendelics Genomic Analysis. Leandro T. Lucato: Lucato receives license fee payments for a lecture from Bracco Imaging—Brazil. He is also an Associate Editor for Neuroimaging since 2010 in Arquivos de Neuropsiquiatria. Fernando Kok: Kok is a shareholder and Medical Director of Mendelics Genomic Analysis and Associated Editor for Neurogenetics of Arquivos de Neuropsiquiatria.
Figures



Similar articles
-
Late diagnosis and atypical brain imaging of Aicardi-Goutières syndrome: are we failing to diagnose Aicardi-Goutières syndrome-2?Dev Med Child Neurol. 2017 Dec;59(12):1307-1311. doi: 10.1111/dmcn.13509. Epub 2017 Aug 1. Dev Med Child Neurol. 2017. PMID: 28762473 Free PMC article.
-
Recurrent Encephalopathy with Spinal Cord Involvement: An Atypical Manifestation of Aicardi-Goutières Syndrome.Ann Indian Acad Neurol. 2019 Jan-Mar;22(1):111-115. doi: 10.4103/aian.AIAN_12_18. Ann Indian Acad Neurol. 2019. PMID: 30692772 Free PMC article.
-
Systemic inflammation and chronic kidney disease in a patient due to the RNASEH2B defect.Pediatr Rheumatol Online J. 2021 Jan 22;19(1):9. doi: 10.1186/s12969-021-00497-2. Pediatr Rheumatol Online J. 2021. PMID: 33482855 Free PMC article.
-
Aicardi-Goutières syndrome: differential diagnosis and aetiopathogenesis.Funct Neurol. 2003 Apr-Jun;18(2):71-5. Funct Neurol. 2003. PMID: 12911136 Review.
-
Aicardi-Goutières syndrome (AGS).Eur J Paediatr Neurol. 2008 Sep;12(5):355-8. doi: 10.1016/j.ejpn.2007.11.010. Epub 2008 Mar 14. Eur J Paediatr Neurol. 2008. PMID: 18343173 Review.
Cited by
-
A decade of whole-exome sequencing in Brazilian Neurology: from past insights to future perspectives.Arq Neuropsiquiatr. 2025 Apr;83(4):1-14. doi: 10.1055/s-0045-1807715. Epub 2025 May 13. Arq Neuropsiquiatr. 2025. PMID: 40360003 Free PMC article. Review.
-
The Rare Syndrome Aicardi-Goutières 4: A Case Report and Literature Review.Dev Neurobiol. 2025 Apr;85(2):e22965. doi: 10.1002/dneu.22965. Dev Neurobiol. 2025. PMID: 40263931 Free PMC article. Review.
-
Editorial for Brain Sciences Special Issue: "Neurogenetic Disorders across Human Life: From Infancy to Adulthood".Brain Sci. 2024 Jan 23;14(2):111. doi: 10.3390/brainsci14020111. Brain Sci. 2024. PMID: 38391686 Free PMC article.
References
LinkOut - more resources
Full Text Sources

