Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion
- PMID: 37626535
- PMCID: PMC10452199
- DOI: 10.3390/brainsci13081179
Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion
Abstract
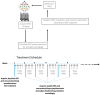
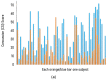
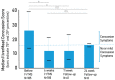
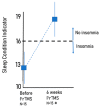
There are no FDA-approved treatments for the chronic sequelae of concussion. Repetitive magnetic transcranial stimulation (rTMS) has been explored as a therapy but outcomes have been inconsistent. To address this we developed a personalized rTMS (PrTMS) protocol involving continual rTMS stimulus frequency adjustment and progressive activation of multiple cortical sites, guided by spectral electroencephalogram (EEG)-based analyses and psychological questionnaires. We acquired pilot clinical data for 185 symptomatic brain concussion patients who underwent the PrTMS protocol over an approximate 6 week period. The PrTMS protocol used a proprietary EEG spectral frequency algorithm to define an initial stimulation frequency based on an anteriorly graded projection of the measured occipital alpha center peak, which was then used to interpolate and adjust regional stimulation frequency according to weekly EEG spectral acquisitions. PrTMS improved concussion indices and normalized the cortical alpha band center frequency and peak EEG amplitude. This potentially reflected changed neurotransmitter, cognitive, and perceptual status. PrTMS may be a promising treatment choice for patients with persistent concussion symptoms. This clinical observational study was limited in that there was no control group and a number of variables were not recorded, such as time since injury and levels of depression. While the present observations are indeed preliminary and cursory, they may suggest further prospective research on PrTMS in concussion, and exploration of the spectral EEG as a concussion biomarker, with the ultimate goals of confirmation and determining optimal PrTMS treatment parameters.
Keywords: Rivermead Post Concussion Symptoms Questionnaire (RPQ); concussion; electroencephalogram (EEG); power spectrum; regression; repetitive transcranial magnetic stimulation (rTMS).
Conflict of interest statement
Murphy owns shares in PeakLogic Inc., Makale receives salary compensation from PeakLogic, Nybo is a founder/owner of CrossTx, Keifer is the owner/operator of BrainHealth Hawaii Incorporated. Blum is Executive Chairman of TranspliceGen Therapeutics Inc., a company that has been licensed to develop his entire patent portfolio including genetic testing and pro-dopamine regulation.
Figures


Similar articles
-
EEG-Spectra-Guided Personalized rTMS in PTSD with Co-occurring Psychiatric Disorders: A Case Series.Acta Sci Neurol. 2025 Jan 24;8(2):3-9. Acta Sci Neurol. 2025. PMID: 40129500 Free PMC article.
-
Pilot Study of Personalized Transcranial Magnetic Stimulation with Spectral Electroencephalogram Analyses for Assessing and Treating Persons with Autism.J Pers Med. 2024 Aug 12;14(8):857. doi: 10.3390/jpm14080857. J Pers Med. 2024. PMID: 39202048 Free PMC article.
-
Personalized repetitive transcranial magnetic stimulation (prtms®) for post-traumatic stress disorder (ptsd) in military combat veterans.Heliyon. 2023 Aug 8;9(8):e18943. doi: 10.1016/j.heliyon.2023.e18943. eCollection 2023 Aug. Heliyon. 2023. PMID: 37609394 Free PMC article.
-
[Interactive rTMS protocols in psychiatry].Encephale. 2013 Dec;39(6):426-31. doi: 10.1016/j.encep.2013.04.009. Epub 2013 Jun 28. Encephale. 2013. PMID: 23810752 Review. French.
-
Use of Repetitive Transcranial Magnetic Stimulation in the Treatment of Neuropsychiatric and Neurocognitive Symptoms Associated With Concussion in Military Populations.J Head Trauma Rehabil. 2020 Nov/Dec;35(6):388-400. doi: 10.1097/HTR.0000000000000628. J Head Trauma Rehabil. 2020. PMID: 33165152 Free PMC article. Review.
Cited by
-
Coupling Bio-Resonance Neurotechnology (BRNT) and Dual Hemispheric Repetitive Transcranial Magnetic Stimulation (rTMS) Reduces Comorbid Major Depressive Disorder (MDD) and Generalized Anxiety Disorder (GAD) as Demonstrated by PHQ-9 and GAD-7: Pilot Case Series.Psychol Res Behav Manag. 2025 Feb 1;18:225-240. doi: 10.2147/PRBM.S482960. eCollection 2025. Psychol Res Behav Manag. 2025. PMID: 39911857 Free PMC article.
-
EEG-Spectra-Guided Personalized rTMS in PTSD with Co-occurring Psychiatric Disorders: A Case Series.Acta Sci Neurol. 2025 Jan 24;8(2):3-9. Acta Sci Neurol. 2025. PMID: 40129500 Free PMC article.
-
A GWAS Meta-meta-analysis and In-depth Silico Pharmacogenomic Investigations in Identification of APOE and Other Genes Associated with Pain, Anti-inflammatory, and Immunomodulating Agents in Opioid Use Disorder (OUD) Derived from 14.91 M Subjects.Cell Mol Neurobiol. 2025 Jul 31;45(1):76. doi: 10.1007/s10571-025-01587-5. Cell Mol Neurobiol. 2025. PMID: 40742457 Free PMC article.
-
Pilot Study of Personalized Transcranial Magnetic Stimulation with Spectral Electroencephalogram Analyses for Assessing and Treating Persons with Autism.J Pers Med. 2024 Aug 12;14(8):857. doi: 10.3390/jpm14080857. J Pers Med. 2024. PMID: 39202048 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

