The Impact of Muscarinic Antagonism on Psychosis-Relevant Behaviors and Striatal [11C] Raclopride Binding in Tau Mouse Models of Alzheimer's Disease
- PMID: 37626588
- PMCID: PMC10452133
- DOI: 10.3390/biomedicines11082091
The Impact of Muscarinic Antagonism on Psychosis-Relevant Behaviors and Striatal [11C] Raclopride Binding in Tau Mouse Models of Alzheimer's Disease
Abstract
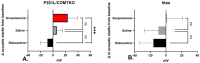
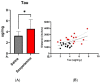
Psychosis that occurs over the course of Alzheimer's disease (AD) is associated with increased caregiver burden and a more rapid cognitive and functional decline. To find new treatment targets, studies modeling psychotic conditions traditionally employ agents known to induce psychosis, utilizing outcomes with cross-species relevance, such as locomotive activity and sensorimotor gating, in rodents. In AD, increased burdens of tau pathology (a diagnostic hallmark of the disease) and treatment with anticholinergic medications have, separately, been reported to increase the risk of psychosis. Recent evidence suggests that muscarinic antagonists may increase extracellular tau. Preclinical studies in AD models have not previously utilized muscarinic cholinergic antagonists as psychotomimetic agents. In this report, we utilize a human-mutant-tau model (P301L/COMTKO) and an over-expressed non-mutant human tau model (htau) in order to compare the impact of antimuscarinic (scopolamine 10 mg/kg/day) treatment with dopaminergic (reboxetine 20 mg/kg/day) treatment, for 7 days, on locomotion and sensorimotor gating. Scopolamine increased spontaneous locomotion, while reboxetine reduced it; neither treatment impacted sensorimotor gating. In the P301L/COMTKO, scopolamine treatment was associated with decreased muscarinic M4 receptor expression, as quantified with RNA-seq, as well as increased dopamine receptor D2 signaling, as estimated with Micro-PET [11C] raclopride binding. Scopolamine also increased soluble tau in the striatum, an effect that partially mediated the observed increases in locomotion. Studies of muscarinic agonists in preclinical tau models are warranted to determine the impact of treatment-on both tau and behavior-that may have relevance to AD and other tauopathies.
Keywords: Alzheimer’s disease; hopping; locomotion; psychosis; scopolamine; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

