Recent Advancements in AAV-Vectored Immunoprophylaxis in the Nonhuman Primate Model
- PMID: 37626720
- PMCID: PMC10452516
- DOI: 10.3390/biomedicines11082223
Recent Advancements in AAV-Vectored Immunoprophylaxis in the Nonhuman Primate Model
Abstract
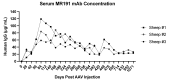
Monoclonal antibodies (mAbs) are important treatment modalities for preventing and treating infectious diseases, especially for those lacking prophylactic vaccines or effective therapies. Recent advances in mAb gene cloning from naturally infected or immunized individuals has led to the development of highly potent human mAbs against a wide range of human and animal pathogens. While effective, the serum half-lives of mAbs are quite variable, with single administrations usually resulting in short-term protection, requiring repeated doses to maintain therapeutic concentrations for extended periods of time. Moreover, due to their limited time in circulation, mAb therapies are rarely given prophylactically; instead, they are generally administered therapeutically after the onset of symptoms, thus preventing mortality, but not morbidity. Adeno-associated virus (AAV) vectors have an established record of high-efficiency in vivo gene transfer in a variety of animal models and humans. When delivered to post-mitotic tissues such as skeletal muscle, brain, and heart, or to organs in which cells turn over slowly, such as the liver and lungs, AAV vector genomes assume the form of episomal concatemers that direct transgene expression, often for the lifetime of the cell. Based on these attributes, many research groups have explored AAV-vectored delivery of highly potent mAb genes as a strategy to enable long-term expression of therapeutic mAbs directly in vivo following intramuscular or intranasal administration. However, clinical trials in humans and studies in nonhuman primates (NHPs) indicate that while AAVs are a powerful and promising platform for vectored immunoprophylaxis (VIP), further optimization is needed to decrease anti-drug antibody (ADA) and anti-capsid antibody responses, ultimately leading to increased serum transgene expression levels and improved therapeutic efficacy. The following review will summarize the current landscape of AAV VIP in NHP models, with an emphasis on vector and transgene design as well as general delivery system optimization. In addition, major obstacles to AAV VIP, along with implications for clinical translation, will be discussed.
Keywords: adeno-associated virus (AAV) vectors; human immunodeficiency virus (HIV); infectious diseases; nonhuman primate (NHP) model; passive immunization; therapeutic monoclonal antibodies; vectored immunoprophylaxis (VIP).
Conflict of interest statement
The funders had no role in the design of the review, in the writing of the manuscript, or in the decision to publish. S.K.W. and B.T. are cofounders of Avamab Pharma Inc., a preclinical, pre-revenue stage company dedicated to the research and development of AAV gene therapies for the treatment and prevention of infectious diseases. S.K.W. is an inventor on issued patents in Canada and the US for the AAV6.2FF capsid, which are owned by the University of Guelph and licensed to Avamab Pharma Inc. From 2020 to February 2023, S.K.W. was an unpaid scientific advisor for Cellastra Inc., which is dedicated to the research and development of gene therapies targeting the root causes of scarring. The authors declare no conflict of interest.
Figures
References
-
- Amanna I.J., Slifka M.K. Successful Vaccines. In: Hangartner L., Burton D.R., editors. Vaccination Strategies Against Highly Variable Pathogens. Volume 428. Springer International Publishing; Cham, Switzerland: 2018. pp. 1–30. Current Topics in Microbiology and Immunology.
-
- Welles H.C., Jennewein M.F., Mason R.D., Narpala S., Wang L., Cheng C., Zhang Y., Todd J.-P., Lifson J.D., Balazs A.B., et al. Vectored Delivery of Anti-SIV Envelope Targeting MAb via AAV8 Protects Rhesus Macaques from Repeated Limiting Dose Intrarectal Swarm SIVsmE660 Challenge. PLoS Pathog. 2018;14:e1007395. doi: 10.1371/journal.ppat.1007395. - DOI - PMC - PubMed
-
- Marcotte H., Hammarström L. Mucosal Immunology. Elsevier; Amsterdam, The Netherlands: 2015. Passive Immunization; pp. 1403–1434.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

